Unknown adverse drug reactions from spontaneous reports in a hospital setting: characterization, follow-up, and contribution to the pharmacovigilance system
- PMID: 37492089
- PMCID: PMC10364048
- DOI: 10.3389/fphar.2023.1211786
Unknown adverse drug reactions from spontaneous reports in a hospital setting: characterization, follow-up, and contribution to the pharmacovigilance system
Abstract
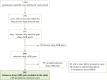
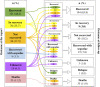
Introduction: Post-marketing identification and report of unknown adverse drug reactions (ADRs) are crucial for patient safety. However, complete information on unknown ADRs seldom is available at the time of spontaneous ADR reports and this can hamper their contribution to the pharmacovigilance system. Methods: In order to characterize the seriousness and outcome of unknown ADRs at the time of report and at follow-up, and analyze their contribution to generate pharmacovigilance regulatory actions, a retrospective observational study of those identified in the spontaneous ADR reports of patients assisted at a hospital (January, 2016-December, 2021) was carried out. Information on demographic, clinical and complementary tests was retrieved from patients' hospital medical records. To evaluate the contribution to pharmacovigilance system we reviewed the European Union SmPCs, the list of the pharmacovigilance signals discussed by the Pharmacovigilance Risk Assessment Committee, and its recommendations reports on safety signals. Results: A total of 15.2% of the spontaneous reported cases during the study contained at least one unknown drug-ADR pair. After exclusions, 295 unknown drug-ADR pairs were included, within them the most frequently affected organs or systems were: skin and subcutaneous tissue (34, 11.5%), hepatobiliary disorders (28, 9.5%), cardiac disorders (28, 9.5%) and central nervous system disorders (27, 9.2%). The most frequent ADRs were pemphigus (7, 2.4%), and cytolytic hepatitis, sudden death, cutaneous vasculitis and fetal growth restriction with 6 (2%) each. Vaccines such as covid-19 and pneumococcus (68, 21.3%), antineoplastics such as paclitaxel, trastuzumab and vincristine (39, 12.2%) and immunosuppressants such as methotrexate and tocilizumab (35, 11%) were the most frequent drug subgroups involved. Sudden death due to hydroxychloroquine alone or in combination (4, 1.4%) and hypertransaminasemia by vincristine (n = 3, 1%) were the most frequent unknown drug-ADR pairs. A total of 269 (91.2%) of them were serious. Complementary tests were performed in 82.7% of unknown-ADR pairs and helped to reinforce their association in 18.3% of them. A total of 18 (6.1%) unknown drug-ADR pairs were evaluated by the EMA, in 8 (2.7%) the information was added to the drug's SmPC and in 1 case the risk prevention material was updated. Conclusion: Identification and follow-up of unknown ADRs can be of great relevance for patient safety and for the enrichment of the pharmacovigilance system.
Keywords: adverse drug reaction reporting systems; drug safety; hospital; patient safety; pharmacovigilance; signal detection; unknown adverse drug reaction.
Copyright © 2023 Filippi-Arriaga, Aguilera, Guillén, Bellas, Pérez, Vendrell, Agustí and Cereza.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Paediatric adverse drug reactions reported to the Spanish Pharmacovigilance System from 2004 to 2009.Eur J Clin Pharmacol. 2012 Sep;68(9):1329-38. doi: 10.1007/s00228-012-1255-0. Epub 2012 Mar 14. Eur J Clin Pharmacol. 2012. PMID: 22415248
-
Evaluation of patient reporting of adverse drug reactions to the UK 'Yellow Card Scheme': literature review, descriptive and qualitative analyses, and questionnaire surveys.Health Technol Assess. 2011 May;15(20):1-234, iii-iv. doi: 10.3310/hta15200. Health Technol Assess. 2011. PMID: 21545758 Review.
-
Adverse Drug Reactions in Children: Comparison of Reports Collected in a Pharmacovigilance Project Versus Spontaneously Collected ADR Reports.Paediatr Drugs. 2023 Mar;25(2):203-215. doi: 10.1007/s40272-022-00540-z. Epub 2022 Nov 12. Paediatr Drugs. 2023. PMID: 36369590 Free PMC article.
-
The contribution of direct patient reported ADRs to drug safety signals in the Netherlands from 2010 to 2015.Pharmacoepidemiol Drug Saf. 2017 Aug;26(8):977-983. doi: 10.1002/pds.4236. Epub 2017 May 19. Pharmacoepidemiol Drug Saf. 2017. PMID: 28524293
-
New Adverse Drug Reaction Signals from 2017 to 2021-Genuine Alerts or False Alarms?Pharmacy (Basel). 2024 Feb 10;12(1):33. doi: 10.3390/pharmacy12010033. Pharmacy (Basel). 2024. PMID: 38392940 Free PMC article. Review.
Cited by
-
Spontaneous adverse drug reactions reported in a thirteen-year pharmacovigilance program in a tertiary university hospital.Front Pharmacol. 2024 Dec 5;15:1427772. doi: 10.3389/fphar.2024.1427772. eCollection 2024. Front Pharmacol. 2024. PMID: 39703397 Free PMC article.
-
Pharmacovigilance - Technological Advancements, Recent Developments and Innovations.Curr Drug Saf. 2025;20(4):423-449. doi: 10.2174/0115748863356840250112181406. Curr Drug Saf. 2025. PMID: 39931995 Review.
-
The Role of Adverse Event Follow-Up in Advancing the Knowledge of Medicines and Vaccines Safety: A Scoping Review.Drug Saf. 2025 Sep;48(9):977-991. doi: 10.1007/s40264-025-01553-6. Epub 2025 May 20. Drug Saf. 2025. PMID: 40392520 Free PMC article. Review.
References
-
- Aguirre C., García M. (2016). Evaluación de la causalidad en las comunicaciones de reacciones adversas a medicamentos. Algoritmo del Sistema Español de Farmacovigilancia. [Causality assessment in reports on adverse drug reactions. Algorithm of Spanish pharmacovigilance system]. Med. Clínica. 147, 461–464. 10.1016/j.medcli.2016.06.012 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials