Effects of bergapten on the pharmacokinetics of macitentan in rats both in vitro and in vivo
- PMID: 37492094
- PMCID: PMC10363979
- DOI: 10.3389/fphar.2023.1204649
Effects of bergapten on the pharmacokinetics of macitentan in rats both in vitro and in vivo
Abstract
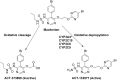
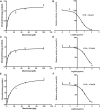
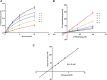
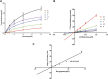
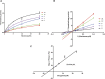
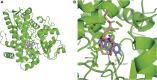
Macitentan was approved by the United States Food and Drug Administration (FDA) in 2013 for the treatment of pulmonary arterial hypertension (PAH). Bergapten is a furanocoumarin that is abundant in Umbelliferae and Rutaceae plants and is widely used in many Chinese medicine prescriptions. Considering the possible combination of these two compounds, this study is aimed to investigate the effects of bergapten on the pharmacokinetics of macitentan both in vitro and in vivo. Rat liver microsomes (RLMs), human liver microsomes (HLMs), and recombinant human CYP3A4 (rCYP3A4) were used to investigate the inhibitory effects and mechanisms of bergapten on macitentan in vitro. In addition, pharmacokinetic parameters were also studied in vivo. Rats were randomly divided into two groups (six rats per group), with or without bergapten (10 mg/kg), and pretreated for 7 days. An oral dose of 20 mg/kg macitentan was administered to each group 30 min after bergapten or 0.5% CMC-Na administration on day 7. Blood was collected from the tail veins, and the plasma concentrations of macitentan and its metabolites were assessed by ultra-performance liquid chromatography - tandem mass spectrometer (UPLC-MS/MS). Finally, we analyzed the binding force of the enzyme and two small ligands by in silico molecular docking to verify the inhibitory effects of bergapten on macitentan. The in vitro results revealed that the IC50 values for RLMs, HLMs, and rCYP3A4 were 3.84, 17.82 and 12.81 μM, respectively. In vivo pharmacokinetic experiments showed that the AUC(0-t), AUC(0-∞), and Cmax of macitentan in the experimental group (20,263.67 μg/L*h, 20,378.31 μg/L*h and 2,999.69 μg/L, respectively) increased significantly compared with the control group (7,873.97 μg/L*h, 7,897.83 μg/L*h and 1,339.44 μg/L, respectively), while the CLz/F (1.07 L/h/kg) of macitentan and the metabolite-parent ratio (MR) displayed a significant decrease. Bergapten competitively inhibited macitentan metabolism in vitro and altered its pharmacokinetic characteristics in vivo. Further molecular docking analysis was also consistent with the experimental results. This study provides a reference for the combined use of bergapten and macitentan in clinical practice.
Keywords: bergapten; drug-drug interaction; macitentan; pharmacokinetics; pulmonary arterial hypertension.
Copyright © 2023 Xu, Zhou, Hou, Wang, Geng, Lu, Zhou, Dai and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Effect of P. corylifolia on the pharmacokinetic profile of tofacitinib and the underlying mechanism.Front Pharmacol. 2024 Apr 8;15:1351882. doi: 10.3389/fphar.2024.1351882. eCollection 2024. Front Pharmacol. 2024. PMID: 38650629 Free PMC article.
-
Drug Interactions of Imperatorin and Curcumin on Macitentan in vitro and in vivo.Drug Des Devel Ther. 2025 Apr 29;19:3459-3475. doi: 10.2147/DDDT.S505960. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40322036 Free PMC article.
-
Effects of Avitinib on CYP450 Enzyme Activity in vitro and in vivo in Rats.Drug Des Devel Ther. 2021 Aug 21;15:3661-3673. doi: 10.2147/DDDT.S323186. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34456561 Free PMC article.
-
Pharmacokinetic and pharmacodynamic evaluation of macitentan , a novel endothelin receptor antagonist for the treatment of pulmonary arterial hypertension.Expert Opin Drug Metab Toxicol. 2015 Mar;11(3):437-49. doi: 10.1517/17425255.2015.1000859. Epub 2015 Jan 21. Expert Opin Drug Metab Toxicol. 2015. PMID: 25604973 Review.
-
Efficacy, safety and clinical pharmacology of macitentan in comparison to other endothelin receptor antagonists in the treatment of pulmonary arterial hypertension.Expert Opin Drug Saf. 2014 Mar;13(3):391-405. doi: 10.1517/14740338.2014.859674. Epub 2013 Nov 22. Expert Opin Drug Saf. 2014. PMID: 24261583 Review.
References
-
- Al-Shakliah N. S., Attwa M. W., Kadi A. A., AlRabiah H. (2020). Identification and characterization of in silico, in vivo, in vitro, and reactive metabolites of infigratinib using LC-ITMS: Bioactivation pathway elucidation and in silico toxicity studies of its metabolites. RSC Adv. 10, 16231–16244. 10.1039/c9ra10871h - DOI - PMC - PubMed
-
- AlRabiah H., Kadi A. A., Attwa M. W., Mostafa G. A. E. (2020). Development and validation of an HPLC-MS/MS method for the determination of filgotinib, a selective Janus kinase 1 inhibitor: Application to a metabolic stability study. J. Chromatogr. B, Anal. Technol. Biomed. life Sci. 1154, 122195. 10.1016/j.jchromb.2020.122195 - DOI - PubMed
LinkOut - more resources
Full Text Sources

