Construction of the systemic anticancer immune environment in tumour-bearing humanized mouse by using liposome-encapsulated anti-programmed death ligand 1 antibody-conjugated progesterone
- PMID: 37492571
- PMCID: PMC10364058
- DOI: 10.3389/fimmu.2023.1173728
Construction of the systemic anticancer immune environment in tumour-bearing humanized mouse by using liposome-encapsulated anti-programmed death ligand 1 antibody-conjugated progesterone
Abstract
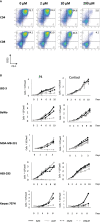
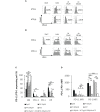
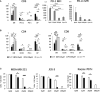
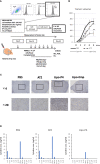
Immune checkpoint inhibitors highlight the importance of anticancer immunity. However, their clinical utility and safety are limited by the low response rates and adverse effects. We focused on progesterone (P4), a hormone produced by the placenta during pregnancy, because it has multiple biological activities related to anticancer and immune regulation effects. P4 has a reversible immune regulatory function distinct from that of the stress hormone cortisol, which may drive irreversible immune suppression that promotes T cell exhaustion and apoptosis in patients with cancer. Because the anticancer effect of P4 is induced at higher than physiological concentrations, we aimed to develop a new anticancer drug by encapsulating P4 in liposomes. In this study, we prepared liposome-encapsulated anti-programmed death ligand 1 (PD-L1) antibody-conjugated P4 (Lipo-anti-PD-L1-P4) and evaluated the effects on the growth of MDA-MB-231 cells, a PD-L1-expressing triple-negative breast cancer cell line, in vitro and in NOG-hIL-4-Tg mice transplanted with human peripheral blood mononuclear cells (humanized mice). Lipo-anti-PD-L1-P4 at physiological concentrations reduced T cell exhaustion and proliferation of MDA-MB-231 in vitro. Humanized mice bearing MDA-MB-231 cells expressing PD-L1 showed suppressed tumor growth and peripheral tissue inflammation. The proportion of B cells and CD4+ T cells decreased, whereas the proportion of CD8+ T cells increased in Lipo-anti-PD-L1-P4-administrated mice spleens and tumor-infiltrated lymphocytes. Our results suggested that Lipo-anti-PD-L1-P4 establishes a systemic anticancer immune environment with minimal toxicity. Thus, the use of P4 as an anticancer drug may represent a new strategy for cancer treatment.
Keywords: breast cancer; humanized mouse; immune environment; liposome; progesterone; programmed death ligand 1.
Copyright © 2023 Kametani, Ito, Ohshima, Manabe, Ohno, Shimizu, Yamada, Katano, Kirigaya, Ito, Matsumoto, Tsuda, Kashiwagi, Goto, Yasuda, Maeki, Tokeshi, Seki, Fukase, Mikami, Ando, Ishimoto and Shiina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

