Are the integrin binding motifs within SARS CoV-2 spike protein and MHC class II alleles playing the key role in COVID-19?
- PMID: 37492575
- PMCID: PMC10364474
- DOI: 10.3389/fimmu.2023.1177691
Are the integrin binding motifs within SARS CoV-2 spike protein and MHC class II alleles playing the key role in COVID-19?
Abstract
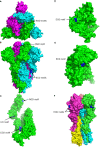
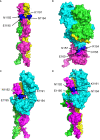
The previous studies on the RGD motif (aa403-405) within the SARS CoV-2 spike (S) protein receptor binding domain (RBD) suggest that the RGD motif binding integrin(s) may play an important role in infection of the host cells. We also discussed the possible role of two other integrin binding motifs that are present in S protein: LDI (aa585-587) and ECD (661-663), the motifs used by some other viruses in the course of infection. The MultiFOLD models for protein structure analysis have shown that the ECD motif is clearly accessible in the S protein, whereas the RGD and LDI motifs are partially accessible. Furthermore, the amino acids that are present in Epstein-Barr virus protein (EBV) gp42 playing very important role in binding to the HLA-DRB1 molecule and in the subsequent immune response evasion, are also present in the S protein heptad repeat-2. Our MultiFOLD model analyses have shown that these amino acids are clearly accessible on the surface in each S protein chain as monomers and in the homotrimer complex and bind to HLA-DRB1 β chain. Therefore, they may have the identical role in SARS CoV-2 immune evasion as in EBV infection. The prediction analyses of the MHC class II binding peptides within the S protein have shown that the RGD motif is present in the core 9-mer peptide IRGDEVRQI within the two HLA-DRB1*03:01 and HLA-DRB3*01.01 strong binding 15-mer peptides suggesting that RGD motif may be the potential immune epitope. Accordingly, infected HLA-DRB1*03:01 or HLA-DRB3*01.01 positive individuals may develop high affinity anti-RGD motif antibodies that react with the RGD motif in the host proteins, like fibrinogen, thrombin or von Willebrand factor, affecting haemostasis or participating in autoimmune disorders.
Keywords: COVID-19; MultiFOLD model; SARS CoV-2 spike protein; autoimmunity; deregulated coagulation; immune evasion; integrin binding motifs.
Copyright © 2023 Gerencer and McGuffin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Can the SARS-CoV-2 Spike Protein Bind Integrins Independent of the RGD Sequence?Front Cell Infect Microbiol. 2021 Nov 18;11:765300. doi: 10.3389/fcimb.2021.765300. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34869067 Free PMC article.
-
SARS-CoV-2 attachment to host cells is possibly mediated via RGD-integrin interaction in a calcium-dependent manner and suggests pulmonary EDTA chelation therapy as a novel treatment for COVID 19.Immunobiology. 2021 Jan;226(1):152021. doi: 10.1016/j.imbio.2020.152021. Epub 2020 Nov 5. Immunobiology. 2021. PMID: 33232865 Free PMC article.
-
Shared Pathogenicity Features and Sequences between EBV, SARS-CoV-2, and HLA Class I Molecule-binding Motifs with a Potential Role in Autoimmunity.Clin Rev Allergy Immunol. 2023 Aug;65(2):206-230. doi: 10.1007/s12016-023-08962-4. Epub 2023 Jul 28. Clin Rev Allergy Immunol. 2023. PMID: 37505416 Review.
-
Biological and Clinical Consequences of Integrin Binding via a Rogue RGD Motif in the SARS CoV-2 Spike Protein.Viruses. 2021 Jan 20;13(2):146. doi: 10.3390/v13020146. Viruses. 2021. PMID: 33498225 Free PMC article. Review.
-
Comprehensive characterization of the antibody responses to SARS-CoV-2 Spike protein finds additional vaccine-induced epitopes beyond those for mild infection.Elife. 2022 Jan 24;11:e73490. doi: 10.7554/eLife.73490. Elife. 2022. PMID: 35072628 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

