Structure, function, and pathology of Neurexin-3
- PMID: 37492720
- PMCID: PMC10363586
- DOI: 10.1016/j.gendis.2022.04.008
Structure, function, and pathology of Neurexin-3
Abstract
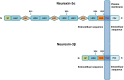
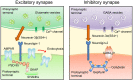
Neurexin-3 is primarily localized in the presynaptic membrane and forms complexes with various ligands located in the postsynaptic membrane. Neurexin-3 has important roles in synapse development and synapse functions. Neurexin-3 mediates excitatory presynaptic differentiation by interacting with leucine-rich-repeat transmembrane neuronal proteins. Meanwhile, neurexin-3 modulates the expression of presynaptic α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors and γ-aminobutyric acid A receptors by interacting with neuroligins at excitatory and inhibitory synapses. Numerous studies have documented the potential contribution of neurexin-3 to neurodegenerative and neuropsychiatric disorders, such as Alzheimer's disease, addiction behaviors, and other diseases, which raises hopes that understanding the mechanisms of neurexin-3 may hold the key to developing new strategies for related illnesses. This review comprehensively covers the literature to provide current knowledge of the structure, function, and clinical role of neurexin-3.
Keywords: Excitatory synapses; Inhibitory synapses; Neural cell adhesion molecules; Neurexin-3; Neurodegenerative diseases; Neuropsychiatric diseases.
© 2022 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Figures

Similar articles
-
SPARCL1 Promotes Excitatory But Not Inhibitory Synapse Formation and Function Independent of Neurexins and Neuroligins.J Neurosci. 2020 Oct 14;40(42):8088-8102. doi: 10.1523/JNEUROSCI.0454-20.2020. Epub 2020 Sep 24. J Neurosci. 2020. PMID: 32973045 Free PMC article.
-
Membrane-tethered monomeric neurexin LNS-domain triggers synapse formation.J Neurosci. 2013 Sep 4;33(36):14617-28. doi: 10.1523/JNEUROSCI.1232-13.2013. J Neurosci. 2013. PMID: 24005312 Free PMC article.
-
Presenilin/γ-secretase regulates neurexin processing at synapses.PLoS One. 2011 Apr 29;6(4):e19430. doi: 10.1371/journal.pone.0019430. PLoS One. 2011. PMID: 21559374 Free PMC article.
-
A matter of balance: role of neurexin and neuroligin at the synapse.Neurochem Res. 2013 Jun;38(6):1174-89. doi: 10.1007/s11064-013-1029-9. Epub 2013 Apr 5. Neurochem Res. 2013. PMID: 23559421 Review.
-
Cerebellin-neurexin complexes instructing synapse properties.Curr Opin Neurobiol. 2023 Aug;81:102727. doi: 10.1016/j.conb.2023.102727. Epub 2023 May 18. Curr Opin Neurobiol. 2023. PMID: 37209532 Review.
Cited by
-
Deciphering novel mitochondrial signatures: multi-omics analysis uncovers cross-disease markers and oligodendrocyte pathways in Alzheimer's disease and glioblastoma.Front Aging Neurosci. 2025 Feb 13;17:1536142. doi: 10.3389/fnagi.2025.1536142. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40018519 Free PMC article.
-
A genome-wide association study of Chinese and English language phenotypes in Hong Kong Chinese children.NPJ Sci Learn. 2024 Mar 27;9(1):26. doi: 10.1038/s41539-024-00229-7. NPJ Sci Learn. 2024. PMID: 38538593 Free PMC article.
-
How do we get from hyperexcitability to excitotoxicity in amyotrophic lateral sclerosis?Brain. 2024 May 3;147(5):1610-1621. doi: 10.1093/brain/awae039. Brain. 2024. PMID: 38408864 Free PMC article. Review.
-
Enhancing therapeutic efficacy in triple-negative breast cancer and melanoma: synergistic effects of modulated electro-hyperthermia (mEHT) with NSAIDs especially COX-2 inhibition in in vivo models.Mol Oncol. 2024 Apr;18(4):1012-1030. doi: 10.1002/1878-0261.13585. Epub 2024 Jan 12. Mol Oncol. 2024. PMID: 38217262 Free PMC article.
-
GIPR agonism and antagonism decrease body weight and food intake via different mechanisms in male mice.Nat Metab. 2025 Jun;7(6):1282-1298. doi: 10.1038/s42255-025-01294-x. Epub 2025 Apr 29. Nat Metab. 2025. PMID: 40301583 Free PMC article.
References
-
- Peineau S., Rabiant K., Pierrefiche O., Potier B. Synaptic plasticity modulation by circulating peptides and metaplasticity: involvement in Alzheimer's disease. Pharmacol Res. 2018;130:385–401. - PubMed
-
- Chih B., Engelman H., Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307(5713):1324–1328. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

