Pseudotyped lentiviral vectors: Ready for translation into targeted cancer gene therapy?
- PMID: 37492721
- PMCID: PMC10363566
- DOI: 10.1016/j.gendis.2022.03.007
Pseudotyped lentiviral vectors: Ready for translation into targeted cancer gene therapy?
Abstract
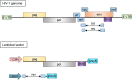
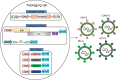
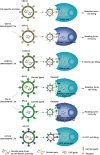
Gene therapy holds great promise for curing cancer by editing the deleterious genes of tumor cells, but the lack of vector systems for efficient delivery of genetic material into specific tumor sites in vivo has limited its full therapeutic potential in cancer gene therapy. Over the past two decades, increasing studies have shown that lentiviral vectors (LVs) modified with different glycoproteins from a donating virus, a process referred to as pseudotyping, have altered tropism and display cell-type specificity in transduction, leading to selective tumor cell killing. This feature of LVs together with their ability to enable high efficient gene delivery in dividing and non-dividing mammalian cells in vivo make them to be attractive tools in future cancer gene therapy. This review is intended to summarize the status quo of some typical pseudotypings of LVs and their applications in basic anti-cancer studies across many malignancies. The opportunities of translating pseudotyped LVs into clinic use in cancer therapy have also been discussed.
Keywords: Cancer therapy; Clinical translation; Gene delivery; Lentiviral vector; Pseudotype.
© 2022 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Figures
Similar articles
-
Selective transduction of astrocytic and neuronal CNS subpopulations by lentiviral vectors pseudotyped with Chikungunya virus envelope.Biomaterials. 2017 Apr;123:1-14. doi: 10.1016/j.biomaterials.2017.01.023. Epub 2017 Jan 23. Biomaterials. 2017. PMID: 28152379
-
Lentiviral Vector Pseudotypes: Precious Tools to Improve Gene Modification of Hematopoietic Cells for Research and Gene Therapy.Viruses. 2020 Sep 11;12(9):1016. doi: 10.3390/v12091016. Viruses. 2020. PMID: 32933033 Free PMC article. Review.
-
Generation of High-Titer Pseudotyped Lentiviral Vectors.Methods Mol Biol. 2019;1937:125-134. doi: 10.1007/978-1-4939-9065-8_7. Methods Mol Biol. 2019. PMID: 30706393
-
Pseudotyped Lentiviral Vectors: One Vector, Many Guises.Hum Gene Ther Methods. 2017 Dec;28(6):291-301. doi: 10.1089/hgtb.2017.084. Epub 2017 Sep 4. Hum Gene Ther Methods. 2017. PMID: 28870117 Review.
-
Optimized Pre-Clinical Grade Production of Two Novel Lentiviral Vector Pseudotypes for Lung Gene Delivery.Hum Gene Ther. 2020 Apr;31(7-8):459-471. doi: 10.1089/hum.2019.211. Epub 2020 Mar 30. Hum Gene Ther. 2020. PMID: 32000531
Cited by
-
Engineering ARMMs for improved intracellular delivery of CRISPR-Cas9.Extracell Vesicle. 2025 Jun;5:100082. doi: 10.1016/j.vesic.2025.100082. Epub 2025 May 9. Extracell Vesicle. 2025. PMID: 40642206 Free PMC article.
-
Optimizing viral transduction in immune cell therapy manufacturing: key process design considerations.J Transl Med. 2025 May 2;23(1):501. doi: 10.1186/s12967-025-06524-0. J Transl Med. 2025. PMID: 40316943 Free PMC article. Review.
-
Progress in Pseudotyping Lentiviral Vectors Towards Cell-Specific Gene Delivery In Vivo.Viruses. 2025 May 31;17(6):802. doi: 10.3390/v17060802. Viruses. 2025. PMID: 40573393 Free PMC article. Review.
-
Utilizing shRNA-expressing lentivectors for viral hemorrhagic septicemia virus suppression via NV gene targeting.Front Vet Sci. 2025 Apr 4;12:1508470. doi: 10.3389/fvets.2025.1508470. eCollection 2025. Front Vet Sci. 2025. PMID: 40256606 Free PMC article.
-
Clinical and Translational Landscape of Viral Gene Therapies.Cells. 2024 Nov 19;13(22):1916. doi: 10.3390/cells13221916. Cells. 2024. PMID: 39594663 Free PMC article. Review.
References
-
- Naldini L. Gene therapy returns to centre stage. Nature. 2015;526(7573):351–360. - PubMed
-
- Dunbar C.E., High K.A., Joung J.K., Kohn D.B., Ozawa K., Sadelain M. Gene therapy comes of age. Science. 2018;359(6372):eaan4672. - PubMed
-
- Ginn S.L., Amaya A.K., Alexander I.E., Edelstein M., Abedi M.R. Gene therapy clinical trials worldwide to 2017: an update. J Gene Med. 2018;20(5):e3015. - PubMed
-
- Poorebrahim M., Sadeghi S., Fakhr E., et al. Production of CAR T-cells by GMP-grade lentiviral vectors: latest advances and future prospects. Crit Rev Clin Lab Sci. 2019;56(6):393–419. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

