Chondroitin Polymerizing Factor (CHPF) promotes cell proliferation and tumor growth in human osteosarcoma by inhibiting SKP2's ubiquitination while activating the AKT pathway
- PMID: 37492722
- PMCID: PMC10363583
- DOI: 10.1016/j.gendis.2022.06.010
Chondroitin Polymerizing Factor (CHPF) promotes cell proliferation and tumor growth in human osteosarcoma by inhibiting SKP2's ubiquitination while activating the AKT pathway
Abstract
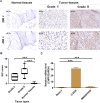
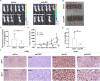
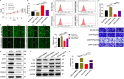
Osteosarcoma is a common malignant tumor occurring in children and young adults. Chondroitin sulfate (CS) participates in cell adhesion, cell division, and the formation of neural networks in the body, the biosynthesis of which requires the participation of glycosyltransferases. CHPF, a glycosyltransferase, plays a role in the extension of CS. Recently, CHPF's biological roles and functional importance in human diseases including malignant tumors have been widely discussed. However, whether CHPF is involved in osteosarcoma development and growth has not been revealed. The present work aimed to investigate the expression levels, functional significance and molecular mechanism of CHPF in osteosarcoma progression. Our results revealed that CHPF is strongly expressed in osteosarcoma tissues and cells. Furthermore, CHPF serves as a tumor promoter in the development and progression of osteosarcoma through enhancing cell proliferation and migration while suppressing apoptosis. Exploration of the mechanism by which CHPF promotes osteosarcoma indicated that CHPF promotes osteosarcoma through counteracting SKP2's ubiquitination and activating the Akt signaling pathway. For the first time, we clarified the roles of CHPF in osteosarcoma, and our results suggested that CHPF might be a novel therapeutic target in the treatment strategies for osteosarcoma.
Keywords: Akt signaling pathway; CHPF; Osteosarcoma; SKP2; Ubiquitination.
© 2022 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Figures


Similar articles
-
Chondroitin polymerizing factor (CHPF) promotes development of malignant melanoma through regulation of CDK1.Cell Death Dis. 2020 Jul 1;11(7):496. doi: 10.1038/s41419-020-2526-9. Cell Death Dis. 2020. PMID: 32612115 Free PMC article.
-
The HNF4A-CHPF pathway promotes proliferation and invasion through interactions with MAD1L1 in glioma.Aging (Albany NY). 2023 Oct 17;15(20):11052-11066. doi: 10.18632/aging.205076. Epub 2023 Oct 17. Aging (Albany NY). 2023. PMID: 37851364 Free PMC article.
-
Chondroitin polymerizing factor (CHPF) contributes to malignant proliferation and migration of hepatocellular carcinoma cells.Biochem Cell Biol. 2020 Jun;98(3):362-369. doi: 10.1139/bcb-2019-0227. Epub 2020 May 8. Biochem Cell Biol. 2020. PMID: 32383983
-
CHPF promotes gastric cancer tumorigenesis through the activation of E2F1.Cell Death Dis. 2021 Sep 25;12(10):876. doi: 10.1038/s41419-021-04148-y. Cell Death Dis. 2021. PMID: 34564711 Free PMC article.
-
Biosynthesis and function of chondroitin sulfate.Biochim Biophys Acta. 2013 Oct;1830(10):4719-33. doi: 10.1016/j.bbagen.2013.06.006. Epub 2013 Jun 14. Biochim Biophys Acta. 2013. PMID: 23774590 Review.
Cited by
-
Ubiquitination in osteosarcoma: unveiling the impact on cell biology and therapeutic strategies.Cancer Biol Med. 2024 Oct 30;21(10):880-97. doi: 10.20892/j.issn.2095-3941.2024.0231. Cancer Biol Med. 2024. PMID: 39475222 Free PMC article. Review.
-
A prognostic glycolysis-related gene signature in osteosarcoma: implications for metabolic programming, immune microenvironment, and drug response.PeerJ. 2025 Apr 29;13:e19369. doi: 10.7717/peerj.19369. eCollection 2025. PeerJ. 2025. PMID: 40321814 Free PMC article.
-
MiR-99a-3p downregulates TRIM21 to promote gastric cancer development.Mol Cell Biochem. 2025 Feb;480(2):1001-1012. doi: 10.1007/s11010-024-05005-0. Epub 2024 May 8. Mol Cell Biochem. 2025. PMID: 38720056 Free PMC article.
References
-
- Sugahara K., Mikami T., Uyama T., et al. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr Opin Struct Biol. 2003;13(5):612–620. - PubMed
-
- Perrimon N., Bernfield M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature. 2000;404(6779):725–728. - PubMed
-
- Sugumaran G., Katsman M., Sunthankar P., et al. Biosynthesis of chondroitin sulfate. Purification of glucuronosyl transferase II and use of photoaffinity labeling for characterization of the enzyme as an 80-kDa protein. J Biol Chem. 1997;272(22):14399–14403. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

