JAG1 enhances angiogenesis in triple-negative breast cancer through promoting the secretion of exosomal lncRNA MALAT1
- PMID: 37492742
- PMCID: PMC10363582
- DOI: 10.1016/j.gendis.2022.07.006
JAG1 enhances angiogenesis in triple-negative breast cancer through promoting the secretion of exosomal lncRNA MALAT1
Abstract
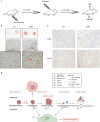
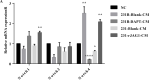
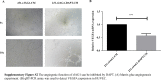
Despite significant improvements in five-year survival rates due to early diagnosis and combination therapy, triple-negative breast cancer (TNBC) treatment remains a major challenge. Finding new and effective targets for diagnosis and drug therapy is urgent for TNBC patients. Jagged-1 (JAG1), one of the canonical ligands of the Notch signaling pathway, is involved in vascular budding and is a poor prognostic factor of TNBC. In this study, combined with quantitative real-time PCR, database analysis, animal experiments, and other means, JAG1 was confirmed to be related to the poor prognosis of TNBC patients. JAG1 was highly expressed in MDA-MB-231 Bone (231B) cells, with stronger invasion and metastasis ability than MDA-MB-231 (231) cells. Treatment of human vascular endothelial cells (HUVEC) with TNBC conditioned medium showed that TNBC JAG1 promoted the angiogenesis of HUVEC. Next, we detected the exosomes extracted from TNBC conditioned medium and found that JAG1 promoted the exosome secretion from 231 cells via ALIX-RAB11A/RAB35. In addition, we also found that the exosomes from JAG1 overexpressed TNBC cells contained more long non-coding RNA (lncRNA) MALAT1, and MALAT1 promoted angiogenesis of HUVEC by targeting miR-140-5p. Finally, the angiogenesis-promoting effect of JAG1 in TNBC was further investigated by matrix gel assay. In conclusion, we reveal that JAG1 has a pro-invasion effect on TNBC and is involved in microenvironment angiogenesis by promoting exosome secretion and the MALAT1-miR-140-5p-JAG1/VEGFA pathway.
Keywords: Angiogenesis; Exosome; JAG1; Triple-negative breast cancer; lncRNAMALAT1.
© 2022 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Conflict of interest statement
We declare that this study was conducted without any commercial or financial relationships.
Figures

Similar articles
-
[JAG1 affects monocytes-macrophages to reshape the pre-metastatic niche of triple-negative breast cancer through LncRNA MALAT1 in exosomes].Nan Fang Yi Ke Da Xue Xue Bao. 2023 Sep 20;43(9):1525-1535. doi: 10.12122/j.issn.1673-4254.2023.09.10. Nan Fang Yi Ke Da Xue Xue Bao. 2023. PMID: 37814867 Free PMC article. Chinese.
-
[JAG1 promotes migration, invasion, and adhesion of triple-negative breast cancer cells by promoting angiogenesis].Nan Fang Yi Ke Da Xue Xue Bao. 2022 Jul 20;42(7):1100-1108. doi: 10.12122/j.issn.1673-4254.2022.07.21. Nan Fang Yi Ke Da Xue Xue Bao. 2022. PMID: 35869777 Free PMC article. Chinese.
-
Long non-coding RNA MALAT1 promotes proliferation and invasion via targeting miR-129-5p in triple-negative breast cancer.Biomed Pharmacother. 2017 Nov;95:922-928. doi: 10.1016/j.biopha.2017.09.005. Epub 2017 Sep 12. Biomed Pharmacother. 2017. PMID: 28915533
-
In-vitro immune-modulation of triple-negative breast cancer through targeting miR-30a-5p/MALAT1 axis using nano-PDT combinational approach.Transl Oncol. 2025 May;55:102365. doi: 10.1016/j.tranon.2025.102365. Epub 2025 Mar 24. Transl Oncol. 2025. PMID: 40132387 Free PMC article.
-
Revolutionizing treatment for triple-negative breast cancer: Harnessing the power of exosomal miRNAs for targeted therapy.Pathol Res Pract. 2023 Oct;250:154825. doi: 10.1016/j.prp.2023.154825. Epub 2023 Sep 16. Pathol Res Pract. 2023. PMID: 37769396 Review.
Cited by
-
Beyond the Genome: Deciphering the Role of MALAT1 in Breast Cancer Progression.Curr Genomics. 2024;25(5):343-357. doi: 10.2174/0113892029305656240503045154. Epub 2024 May 22. Curr Genomics. 2024. PMID: 39323624 Free PMC article. Review.
-
Reduced JAG1 Expression Through miR-200 Overexpression or Crispr-Cas Mediated Knockout Impairs TNBC Growth and Metastasis.Mol Carcinog. 2025 Aug;64(8):1392-1407. doi: 10.1002/mc.23937. Epub 2025 Jun 11. Mol Carcinog. 2025. PMID: 40499559 Free PMC article.
-
Alteration of skin fibroblast steady state contributes to healing outcomes.bioRxiv [Preprint]. 2024 Dec 12:2024.12.06.627278. doi: 10.1101/2024.12.06.627278. bioRxiv. 2024. PMID: 39713414 Free PMC article. Preprint.
-
The Regulation of Exosome Generation and Function in Physiological and Pathological Processes.Int J Mol Sci. 2023 Dec 23;25(1):255. doi: 10.3390/ijms25010255. Int J Mol Sci. 2023. PMID: 38203424 Free PMC article. Review.
-
Notch signaling pathway in cancer: from mechanistic insights to targeted therapies.Signal Transduct Target Ther. 2024 May 27;9(1):128. doi: 10.1038/s41392-024-01828-x. Signal Transduct Target Ther. 2024. PMID: 38797752 Free PMC article. Review.
References
-
- World health organization . 2020. Latest Global Cancer Data: Cancer Burden Rises to 19.3 Million New Cases and 10.0 Million Cancer Deaths in 2020.https://www.iarc.who.int/faq/latest-global-cancer-data-2020-qa/
-
- Gluz O., Liedtke C., Gottschalk N., Pusztai L., Nitz U., Harbeck N. Triple-negative breast cancer: current status and future directions. Ann Oncol. 2009;20(12):1913–1927. - PubMed
-
- Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

