Prevalence and Patient-Level Correlates of Intentional Non-Adherence to Immunosuppressive Medication After Heart-Transplantation-Findings From the International BRIGHT Study
- PMID: 37492859
- PMCID: PMC10363605
- DOI: 10.3389/ti.2023.11308
Prevalence and Patient-Level Correlates of Intentional Non-Adherence to Immunosuppressive Medication After Heart-Transplantation-Findings From the International BRIGHT Study
Abstract
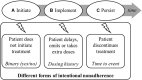
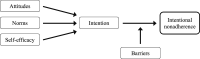
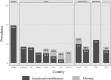
After heart transplantation (HTx), non-adherence to immunosuppressants (IS) is associated with poor outcomes; however, intentional non-adherence (INA) is poorly understood regarding its international variability in prevalence, contributing factors and impact on outcomes. We investigated (1) the prevalence and international variability of INA, (2) patient-level correlates of INA, and (3) relation of INA with clinical outcomes. Secondary analysis of data from the BRIGHT study-an international multi-center, cross-sectional survey examining multi-level factors of adherence in 1,397 adult HTx recipients. INA during the implementation phase, i.e., drug holiday and dose alteration, was measured using the Basel Assessment of Adherence to Immunosuppressive Medications Scale© (BAASIS©). Descriptive and inferential analysis was performed with data retrieved through patient interview, patient self-report and in clinical records. INA prevalence was 3.3% (n = 46/1,397)-drug holidays: 1.7% (n = 24); dose alteration: 1.4% (n = 20); both: 0.1% (n = 2). University-level education (OR = 2.46, CI = 1.04-5.83), insurance not covering IS costs (OR = 2.21, CI = 1.01-4.87) and barriers (OR = 4.90, CI = 2.73-8.80) were significantly associated with INA; however, clinical outcomes were not. Compared to other single-center studies, this sample's INA prevalence was low. More than accessibility or financial concerns, our analyses identified patient-level barriers as INA drivers. Addressing patients' IS-related barriers, should decrease INA.
Keywords: correlates; heart transplantation; immunosuppression; intentional non-adherence; medication non-adherence.
Copyright © 2023 Marston, Berben, Dobbels, Russell and de Geest.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Prevalence and Correlates of Cost-Related Medication Nonadherence to Immunosuppressive Drugs After Heart Transplantation: The International Multicenter Cross-sectional Bright Study.J Cardiovasc Nurs. 2020 Nov/Dec;35(6):519-529. doi: 10.1097/JCN.0000000000000683. J Cardiovasc Nurs. 2020. PMID: 32433348 Free PMC article.
-
Prevalence of Medication Nonadherence to Co-medication Compared to Immunosuppressants in Heart Transplant Recipients: Findings From the International Cross-sectional BRIGHT Study.Clin Ther. 2019 Jan;41(1):130-136. doi: 10.1016/j.clinthera.2018.11.007. Epub 2018 Dec 24. Clin Ther. 2019. PMID: 30591285
-
Multilevel factors are associated with immunosuppressant nonadherence in heart transplant recipients: The international BRIGHT study.Am J Transplant. 2018 Jun;18(6):1447-1460. doi: 10.1111/ajt.14611. Epub 2018 Jan 16. Am J Transplant. 2018. PMID: 29205855 Free PMC article.
-
Prevalence and Risk Factors of Immunosuppressant Nonadherence in Heart Transplant Recipients: A Single-Center Cross-Sectional Study.Patient Prefer Adherence. 2019 Dec 20;13:2185-2193. doi: 10.2147/PPA.S223837. eCollection 2019. Patient Prefer Adherence. 2019. PMID: 31908425 Free PMC article.
-
Non-adherence with drug treatment after heart or lung transplantation in adults: a systematic review.Patient Educ Couns. 2010 Nov;81(2):148-54. doi: 10.1016/j.pec.2010.04.013. Epub 2010 Jun 2. Patient Educ Couns. 2010. PMID: 20627643
Cited by
-
Immunosuppressant nonadherence profile in kidney transplant recipients and the impact of medication adherence on transplant outcomes.Front Pharmacol. 2024 Dec 18;15:1493166. doi: 10.3389/fphar.2024.1493166. eCollection 2024. Front Pharmacol. 2024. PMID: 39744122 Free PMC article.
-
Inconclusiveness of psychometric testing of medication adherence questionnaires.Eur J Clin Pharmacol. 2024 Aug;80(8):1189-1195. doi: 10.1007/s00228-024-03684-8. Epub 2024 Apr 22. Eur J Clin Pharmacol. 2024. PMID: 38647703 Free PMC article.
-
Medication Adherence Barriers and Their Relationship to Health Determinants for Saudi Pediatric Dialysis Patients.Children (Basel). 2024 Feb 29;11(3):293. doi: 10.3390/children11030293. Children (Basel). 2024. PMID: 38539328 Free PMC article.
-
Barriers to Immunosuppressant Medication Adherence in Thoracic Transplant Recipients: Initial Findings.Int J Environ Res Public Health. 2025 Jul 8;22(7):1090. doi: 10.3390/ijerph22071090. Int J Environ Res Public Health. 2025. PMID: 40724157 Free PMC article.
References
-
- De Geest S, Denhaerynck K, Dobbels F. Clinical and Economical Consequences of Nonadherence to Immunosuppressive Drugs in Adult Solid Organ Transplantation (Invited Editor: Dr. Federico Oppenheime). In: Grinyò DJ, editor. International Transplantation Updates 2011. Barcelona, Spain: Permanyer Publications; (2011). p. 63–81.
-
- Horne R, Chapman SC, Parham R, Freemantle N, Forbes A, Cooper V. Understanding Patients' Adherence-Related Beliefs about Medicines Prescribed for Long-Term Conditions: a Meta-Analytic Review of the Necessity-Concerns Framework. PLoS One (2013) 8:e80633. 10.1371/journal.pone.0080633 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

