Insights into acute mesenteric ischaemia: an up-to-date, evidence-based review from a mesenteric stroke centre unit
- PMID: 37493183
- PMCID: PMC10607400
- DOI: 10.1259/bjr.20230232
Insights into acute mesenteric ischaemia: an up-to-date, evidence-based review from a mesenteric stroke centre unit
Abstract
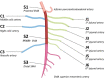
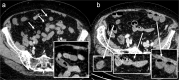
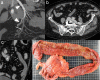
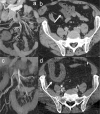
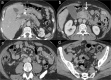
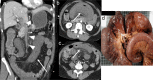
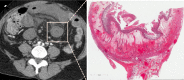
Radiologists play a central role in the diagnostic and prognostic evaluation of patients with acute mesenteric ischaemia (AMI). Unfortunately, more than half of AMI patients undergo imaging with no prior suspicion of AMI, making identifying this disease even more difficult. A confirmed diagnosis of AMI is ideally made with dynamic contrast-enhanced CT but the diagnosis may be made on portal-venous phase images in appropriate clinical settings. AMI is diagnosed on CT based on the identification of vascular impairment and bowel ischaemic injury with no other cause. Moreover, radiologists must evaluate the probability of bowel necrosis, which will influence the treatment options.AMI is usually separated into different entities: arterial, venous, non-occlusive and ischaemic colitis. Arterial AMI can be occlusive or stenotic, the dominant causes being atherothrombosis, embolism and isolated superior mesenteric artery (SMA) dissection. The main finding in the bowel is decreased wall enhancement, and necrosis can be suspected when dilatation >25 mm is identified. Venous AMI is related to superior mesenteric vein (SMV) thrombosis as a result of a thrombophilic state (acquired or inherited), local injury (cancer, inflammation or trauma) or underlying SMV insufficiency. The dominant features in the bowel are hypoattenuating wall thickening with submucosal oedema. Decreased enhancement of the involved bowel suggests necrosis. Non-occlusive mesenteric ischaemia (NOMI) is related to impaired SMA flow following global hypoperfusion associated with low-flow states. There are numerous findings in the bowel characterised by diffuse extension. An absence of bowel enhancement and a thin bowel wall suggest necrosis in NOMI. Finally, ischaemic colitis is a sub-entity of arterial AMI and reflects localised colon ischaemia-reperfusion injury. The main CT finding is a thickened colon wall with fat stranding, which seems to be unrelated to SMA or inferior mesenteric artery lesions. A precise identification and description of vascular lesions, bowel involvement and features associated with transmural necrosis is needed to determine patient treatment and outcome.
Figures


References
-
- Cokkinis AJ. Mesenteric vascular occlusion. Southern Medical Journal 1926; 19: 655. doi: 10.1097/00007611-192608000-00028 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

