Preclinical Development of Brain Permeable ERβ Agonist for the Treatment of Glioblastoma
- PMID: 37493258
- PMCID: PMC10811744
- DOI: 10.1158/1535-7163.MCT-23-0031
Preclinical Development of Brain Permeable ERβ Agonist for the Treatment of Glioblastoma
Abstract
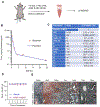
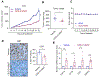
Glioblastoma (GBM) is the most prevalent and aggressive type of adult brain tumors with low 5-year overall survival rates. Epidemiologic data suggest that estrogen may decrease brain tumor growth, and estrogen receptor beta (ERβ) has been demonstrated to exert antitumor functions in GBM. The lack of potent, selective, and brain permeable ERβ agonist to promote its antitumor action is limiting the therapeutic promise of ERβ. In this study, we discovered that Indanone and tetralone-keto or hydroxyl oximes are a new class of ERβ agonists. Because of its high activity in ERβ reporter assays, specific binding to ERβ in polar screen assays, and potent growth inhibitory activity in GBM cells, CIDD-0149897 was discovered as a possible hit by screening a library of compounds. CIDD-0149897 is more selective for ERβ than ERα (40-fold). Treatment with CIDD-0149897 markedly reduced GBM cell viability with an IC50 of ∼7 to 15 μmol/L, while having little to no effect on ERβ-KO cells and normal human astrocytes. Further, CIDD-0149897 treatment enhanced expression of known ERβ target genes and promoted apoptosis in established and patient-derived GSC models. Pharmacokinetic studies confirmed that CIDD-0149897 has systemic exposure, and good bioavailability in the brain. Mice tolerated daily intraperitoneal treatment of CIDD-0149897 (50 mg/kg) with a 7-day repeat dosage with no toxicity. In addition, CIDD-0149897 treatment significantly decreased tumor growth in U251 xenograft model and extended the survival of orthotopic GBM tumor-bearing mice. Collectively, these findings pointed to CIDD-0149897 as a new class of ERβ agonist, offering patients with GBM a potential means of improving survival.
©2023 American Association for Cancer Research.
Conflict of interest statement
Figures





References
-
- Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017;318(23):2306–16 doi 10.1001/jama.2017.18718. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

