NLRP14 Safeguards Calcium Homeostasis via Regulating the K27 Ubiquitination of Nclx in Oocyte-to-Embryo Transition
- PMID: 37493331
- PMCID: PMC10520637
- DOI: 10.1002/advs.202301940
NLRP14 Safeguards Calcium Homeostasis via Regulating the K27 Ubiquitination of Nclx in Oocyte-to-Embryo Transition
Abstract
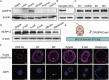
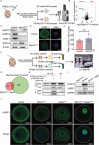
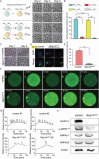
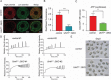
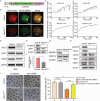
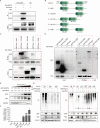
Sperm-induced Ca2+ rise is critical for driving oocyte activation and subsequent embryonic development, but little is known about how lasting Ca2+ oscillations are regulated. Here it is shown that NLRP14, a maternal effect factor, is essential for keeping Ca2+ oscillations and early embryonic development. Few embryos lacking maternal NLRP14 can develop beyond the 2-cell stage. The impaired developmental potential of Nlrp14-deficient oocytes is mainly caused by disrupted cytoplasmic function and calcium homeostasis due to altered mitochondrial distribution, morphology, and activity since the calcium oscillations and development of Nlrp14-deficient oocytes can be rescued by substitution of whole cytoplasm by spindle transfer. Proteomics analysis reveal that cytoplasmic UHRF1 (ubiquitin-like, containing PHD and RING finger domains 1) is significantly decreased in Nlrp14-deficient oocytes, and Uhrf1-deficient oocytes also show disrupted calcium homeostasis and developmental arrest. Strikingly, it is found that the mitochondrial Na+ /Ca2+ exchanger (NCLX) encoded by Slc8b1 is significantly decreased in the Nlrp14mNull oocyte. Mechanistically, NLRP14 interacts with the NCLX intrinsically disordered regions (IDRs) domain and maintain its stability by regulating the K27-linked ubiquitination. Thus, the study reveals NLRP14 as a crucial player in calcium homeostasis that is important for early embryonic development.
Keywords: NCLX; NLRP14; UHRF1; adenosine triphosphate (ATP); calcium homeostasis; early embryonic development; maternal effect genes; mitochondria.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Brower P. T., Gizang E., Boreen S. M., Schultz R. M., Developmental Biology 1981, 86, 373. - PubMed
-
- a) Tong Z.‐B., Gold L., Pfeifer K. E., Dorward H., Lee E., Bondy C. A., Dean J., Nelson L. M., Nat. Genet. 2000, 26, 267; - PubMed
- b) Esposito G., Vitale A. M., Leijten F. P., Strik A. M., Koonen‐Reemst A. M., Yurttas P., Robben T. J., Coonrod S., Gossen J. A., Mol. Cell. Endocrinol. 2007, 273, 25; - PubMed
- c) Payer B., Saitou M., Barton S. C., Thresher R., Dixon J. P., Zahn D., Colledge W. H., Carlton M. B., Nakano T., Surani M. A., Curr Biol 2003, 13, 2110. - PubMed
-
- a) Hamatani T., Falco G., Carter M. G., Akutsu H., Stagg C. A., Sharov A. A., Dudekula D. B., VanBuren V., Ko M. S. H., Hum. Mol. Genet. 2004, 13, 2263; - PubMed
- b) Sharov A. A., Falco G., Piao Y., Poosala S., Becker K. G., Zonderman A. B., Longo D. L., Schlessinger D., Ko M., BMC Biol. 2008, 6, 24. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
