Safety and Efficacy of Liraglutide, 3.0 mg, Once Daily vs Placebo in Patients With Poor Weight Loss Following Metabolic Surgery: The BARI-OPTIMISE Randomized Clinical Trial
- PMID: 37494014
- PMCID: PMC10372755
- DOI: 10.1001/jamasurg.2023.2930
Safety and Efficacy of Liraglutide, 3.0 mg, Once Daily vs Placebo in Patients With Poor Weight Loss Following Metabolic Surgery: The BARI-OPTIMISE Randomized Clinical Trial
Abstract
Importance: Metabolic surgery leads to weight loss and improved health, but these outcomes are highly variable. Poor weight loss is associated with lower circulating levels of glucagon-like peptide-1 (GLP-1).
Objective: To assess the efficacy and safety of the GLP-1 receptor agonist, liraglutide, 3.0 mg, on percentage body weight reduction in patients with poor weight loss and suboptimal GLP-1 response after metabolic surgery.
Design, setting, and participants: The Evaluation of Liraglutide 3.0 mg in Patients With Poor Weight Loss and a Suboptimal Glucagon-Like Peptide-1 Response (BARI-OPTIMISE) randomized placebo-controlled trial recruited adult patients at least 1 year after metabolic surgery who had experienced 20% or less body weight loss from the day of surgery and a suboptimal nutrient-stimulated GLP-1 response from 2 hospitals in London, United Kingdom, between October 2018 and November 2019. Key exclusion criteria were type 1 diabetes; severe concomitant psychiatric, gastrointestinal, cardiac, kidney or metabolic disease; and use of insulin, GLP-1 receptor analogues, and medication that can affect weight. The study period was 24 weeks followed by a 4-week follow-up period. Last participant follow-up was completed in June 2020. All participants and clinical study personnel were blinded to treatment allocation. Of 154 assessed for eligibility, 70 met trial criteria and were included in the study, and 57 completed follow-up.
Interventions: Liraglutide, 3.0 mg, once daily or placebo as an adjunct to lifestyle intervention with a 500-kcal daily energy deficit for 24 weeks, on a 1:1 allocation by computer-generated randomization sequence, stratified by surgery type (Roux-en-Y gastric bypass [RYGB] or sleeve gastrectomy [SG]) and type 2 diabetes status.
Main outcome and measures: The primary outcome was change in percentage body weight from baseline to the end of the 24-week study period based on an intention-to-treat analysis. Participant safety was assessed through monitoring of biochemical parameters, including kidney and liver function, physical examination, and assessment for adverse events.
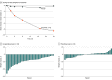
Results: A total of 70 participants (mean [SD] age, 47.6 [10.7] years; 52 [74%] female) with a poor weight loss response following RYGB or SG were randomized to receive 3.0-mg liraglutide (n = 35) or placebo (n = 35). All participants received at least 1 dose of the trial drug. Eight participants discontinued treatment (4 per group), and 2 in the 3.0-mg liraglutide group and 1 in the placebo group were lost to follow-up. Due to COVID-19 restrictions, 3 participants in the 3.0-mg liraglutide group and 7 in the placebo group were unable to attend their final in-person assessment. Estimated change in mean (SD) percentage body weight from baseline to week 24 was -8.82 (4.94) with liraglutide, 3.0 mg (n = 31), vs -0.54 (3.32) with placebo (n = 26). The mean difference in percentage body weight change for liraglutide, 3.0 mg, vs placebo was -8.03 (95% CI, -10.39 to -5.66; P < .001). Adverse events, predominantly gastrointestinal, were more frequent with liraglutide, 3.0 mg (28 events [80%]), than placebo (20 events [57%]). There were no serious adverse events and no treatment-related deaths.
Conclusion and relevance: These findings support the use of adjuvant liraglutide, 3.0 mg, for weight management in patients with poor weight loss and suboptimal GLP-1 response after metabolic surgery.
Trial registration: ClinicalTrials.gov Identifier: NCT03341429.
Conflict of interest statement
Figures
Comment in
-
Treatment of Severe Obesity-All-Hands-on-Deck Approach.JAMA Surg. 2023 Oct 1;158(10):1011-1012. doi: 10.1001/jamasurg.2023.2931. JAMA Surg. 2023. PMID: 37494032 No abstract available.
References
-
- Schauer PR, Bhatt DL, Kashyap SR. Bariatric surgery or intensive medical therapy for diabetes after 5 years. N Engl J Med. 2017;376(20):1997. - PubMed
-
- Syn NL, Cummings DE, Wang LZ, et al. . Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. 2021;397(10287):1830-1841. doi:10.1016/S0140-6736(21)00591-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

