Low-Dose Aspirin and the Risk of Stroke and Intracerebral Bleeding in Healthy Older People: Secondary Analysis of a Randomized Clinical Trial
- PMID: 37494038
- PMCID: PMC10372701
- DOI: 10.1001/jamanetworkopen.2023.25803
Low-Dose Aspirin and the Risk of Stroke and Intracerebral Bleeding in Healthy Older People: Secondary Analysis of a Randomized Clinical Trial
Erratum in
-
Incorrect Data in Results and Figures.JAMA Netw Open. 2023 Oct 2;6(10):e2340464. doi: 10.1001/jamanetworkopen.2023.40464. JAMA Netw Open. 2023. PMID: 37843867 Free PMC article. No abstract available.
Abstract
Importance: Low-dose aspirin has been widely used for primary and secondary prevention of stroke. The balance between potential reduction of ischemic stroke events and increased intracranial bleeding has not been established in older individuals.
Objective: To establish the risks of ischemic stroke and intracranial bleeding among healthy older people receiving daily low-dose aspirin.
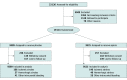
Design, setting, and participants: This secondary analysis of the Aspirin in Reducing Events in the Elderly (ASPREE) randomized, double-blind, placebo-controlled trial of daily low-dose aspirin was conducted among community-dwelling people living in Australia or the US. Participants were older adults free of symptomatic cardiovascular disease. Recruitment took place between 2010 and 2014, and participants were followed up for a median (IQR) of 4.7 (3.6-5.7) years. This analysis was completed from August 2021 to March 2023.
Interventions: Daily 100-mg enteric-coated aspirin or matching placebo.
Main outcomes and measures: Stroke and stroke etiology were predetermined secondary outcomes and are presented with a focus on prevention of initial stroke or intracranial bleeding event. Outcomes were assessed by review of medical records.
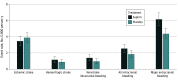
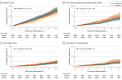
Results: Among 19 114 older adults (10 782 females [56.4%]; median [IQR] age, 74 [71.6-77.7] years), 9525 individuals received aspirin and 9589 individuals received placebo. Aspirin did not produce a statistically significant reduction in the incidence of ischemic stroke (hazard ratio [HR], 0.89; 95% CI, 0.71-1.11). However, a statistically significant increase in intracranial bleeding was observed among individuals assigned to aspirin (108 individuals [1.1%]) compared with those receiving placebo (79 individuals [0.8%]; HR, 1.38; 95% CI, 1.03-1.84). This occurred by an increase in a combination of subdural, extradural, and subarachnoid bleeding with aspirin compared with placebo (59 individuals [0.6%] vs 41 individuals [0.4%]; HR, 1.45; 95% CI, 0.98-2.16). Hemorrhagic stroke was recorded in 49 individuals (0.5%) assigned to aspirin compared with 37 individuals (0.4%) in the placebo group (HR, 1.33; 95% CI, 0.87-2.04).
Conclusions and relevance: This study found a significant increase in intracranial bleeding with daily low-dose aspirin but no significant reduction of ischemic stroke. These findings may have particular relevance to older individuals prone to developing intracranial bleeding after head trauma.
Trial registration: ISRCTN.org Identifier: ISRCTN83772183.
Conflict of interest statement
Figures

Comment in
-
In healthy older adults, low-dose aspirin did not differ from placebo for ischemic stroke but increased intracranial bleeding.Ann Intern Med. 2023 Nov;176(11):JC126. doi: 10.7326/J23-0092. Epub 2023 Nov 7. Ann Intern Med. 2023. PMID: 37931254
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

