Searching for molecular hypoxia sensors among oxygen-dependent enzymes
- PMID: 37494095
- PMCID: PMC10371230
- DOI: 10.7554/eLife.87705
Searching for molecular hypoxia sensors among oxygen-dependent enzymes
Abstract
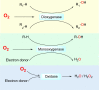
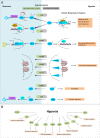
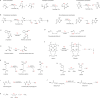
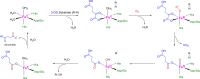
The ability to sense and respond to changes in cellular oxygen levels is critical for aerobic organisms and requires a molecular oxygen sensor. The prototypical sensor is the oxygen-dependent enzyme PHD: hypoxia inhibits its ability to hydroxylate the transcription factor HIF, causing HIF to accumulate and trigger the classic HIF-dependent hypoxia response. A small handful of other oxygen sensors are known, all of which are oxygen-dependent enzymes. However, hundreds of oxygen-dependent enzymes exist among aerobic organisms, raising the possibility that additional sensors remain to be discovered. This review summarizes known and potential hypoxia sensors among human O2-dependent enzymes and highlights their possible roles in hypoxia-related adaptation and diseases.
Keywords: cell biology; hypoxia; hypoxia sensors; oxygen; oxygen-dependent enzymes.
© 2023, Li et al.
Conflict of interest statement
LL, SS, PB, MJ, LW, SA No competing interests declared
Figures
Similar articles
-
Intracellular localisation of human HIF-1 alpha hydroxylases: implications for oxygen sensing.J Cell Sci. 2003 Apr 1;116(Pt 7):1319-26. doi: 10.1242/jcs.00318. J Cell Sci. 2003. PMID: 12615973
-
Molecular evolution of the metazoan PHD-HIF oxygen-sensing system.Mol Biol Evol. 2011 Jun;28(6):1913-26. doi: 10.1093/molbev/msr012. Epub 2011 Jan 12. Mol Biol Evol. 2011. PMID: 21228399
-
PHD1-3 oxygen sensors in vivo-lessons learned from gene deletions.Pflugers Arch. 2024 Sep;476(9):1307-1337. doi: 10.1007/s00424-024-02944-x. Epub 2024 Mar 21. Pflugers Arch. 2024. PMID: 38509356 Free PMC article. Review.
-
HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia.EMBO J. 2003 Aug 15;22(16):4082-90. doi: 10.1093/emboj/cdg392. EMBO J. 2003. PMID: 12912907 Free PMC article.
-
The hypoxia-inducible-factor hydroxylases bring fresh air into hypoxia signalling.EMBO Rep. 2006 Jan;7(1):41-5. doi: 10.1038/sj.embor.7400598. EMBO Rep. 2006. PMID: 16391536 Free PMC article. Review.
Cited by
-
Hypoxia inducible factor-1ɑ as a potential therapeutic target for osteosarcoma metastasis.Front Pharmacol. 2024 Jan 24;15:1350187. doi: 10.3389/fphar.2024.1350187. eCollection 2024. Front Pharmacol. 2024. PMID: 38327979 Free PMC article. Review.
-
A phenopushing platform to identify compounds that alleviate acute hypoxic stress by fast-tracking cellular adaptation.Nat Commun. 2025 Mar 18;16(1):2684. doi: 10.1038/s41467-025-57754-1. Nat Commun. 2025. PMID: 40102413 Free PMC article.
-
Hif1α-dependent mitochondrial acute O2 sensing and signaling to myocyte Ca2+ channels mediate arterial hypoxic vasodilation.Nat Commun. 2024 Aug 5;15(1):6649. doi: 10.1038/s41467-024-51023-3. Nat Commun. 2024. PMID: 39103356 Free PMC article.
-
Gene expression and DNA methylation changes in response to hypoxia in toxicant-adapted Atlantic killifish (Fundulus heteroclitus).Biol Open. 2025 Jan 15;14(1):BIO061801. doi: 10.1242/bio.061801. Epub 2025 Jan 6. Biol Open. 2025. PMID: 39760289 Free PMC article.
-
The presence of substrate warrants oxygen access tunnels toward the catalytic site of lipoxygenases.Redox Biol. 2025 Jun;83:103636. doi: 10.1016/j.redox.2025.103636. Epub 2025 Apr 11. Redox Biol. 2025. PMID: 40245701 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

