Whole Genome Duplication and Gene Evolution in the Hyperdiverse Venomous Gastropods
- PMID: 37494290
- PMCID: PMC10401626
- DOI: 10.1093/molbev/msad171
Whole Genome Duplication and Gene Evolution in the Hyperdiverse Venomous Gastropods
Abstract
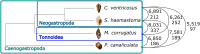
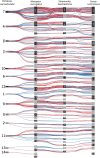
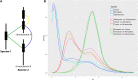
The diversity of venomous organisms and the toxins they produce have been increasingly investigated, but taxonomic bias remains important. Neogastropods, a group of marine predators representing almost 22% of the known gastropod diversity, evolved a wide range of feeding strategies, including the production of toxins to subdue their preys. However, whether the diversity of these compounds is at the origin of the hyperdiversification of the group and how genome evolution may correlate with both the compounds and species diversities remain understudied. Among the available gastropods genomes, only eight, with uneven quality assemblies, belong to neogastropods. Here, we generated chromosome-level assemblies of two species belonging to the Tonnoidea and Muricoidea superfamilies (Monoplex corrugatus and Stramonita haemastoma). The two obtained high-quality genomes had 3 and 2.2 Gb, respectively, and 92-89% of the total assembly conformed 35 pseudochromosomes in each species. Through the analysis of syntenic blocks, Hox gene cluster duplication, and synonymous substitutions distribution pattern, we inferred the occurrence of a whole genome duplication event in both genomes. As these species are known to release venom, toxins were annotated in both genomes, but few of them were found in homologous chromosomes. A comparison of the expression of ohnolog genes (using transcriptomes from osphradium and salivary glands in S. haemastoma), where both copies were differentially expressed, showed that most of them had similar expression profiles. The high quality of these genomes makes them valuable reference in their respective taxa, facilitating the identification of genome-level processes at the origin of their evolutionary success.
Keywords: genomes; neofunctionalization; neogastropods; venom; whole-genome duplication.
© The Author(s) 2023. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Figures


References
-
- Almeida DD, Viala VL, Nachtigall PG, Broe M, Gibbs HL, Serrano SMT, Moura-da-Silva AM, Ho PL, Nishiyama-Jr MY, Junqueira-de-Azevedo ILM. 2021. Tracking the recruitment and evolution of snake toxins using the evolutionary context provided by the Bothrops jararaca genome. Proc Natl Acad Sci. 118:e2015159118. - PMC - PubMed
-
- Balaji RA, Ohtake A, Sato K, Gopalakrishnakone P, Kini RM, Seow KT, Bay B-H. 2000. λ-Conotoxins, a new family of conotoxins with unique disulfide pattern and protein folding. J Biol Chem. 275:39516–39522. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

