Assessment of stored red blood cells through lab-on-a-chip technologies for precision transfusion medicine
- PMID: 37494421
- PMCID: PMC10410732
- DOI: 10.1073/pnas.2115616120
Assessment of stored red blood cells through lab-on-a-chip technologies for precision transfusion medicine
Abstract
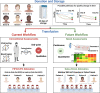
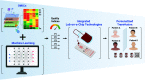
Transfusion of red blood cells (RBCs) is one of the most valuable and widespread treatments in modern medicine. Lifesaving RBC transfusions are facilitated by the cold storage of RBC units in blood banks worldwide. Currently, RBC storage and subsequent transfusion practices are performed using simplistic workflows. More specifically, most blood banks follow the "first-in-first-out" principle to avoid wastage, whereas most healthcare providers prefer the "last-in-first-out" approach simply favoring chronologically younger RBCs. Neither approach addresses recent advances through -omics showing that stored RBC quality is highly variable depending on donor-, time-, and processing-specific factors. Thus, it is time to rethink our workflows in transfusion medicine taking advantage of novel technologies to perform RBC quality assessment. We imagine a future where lab-on-a-chip technologies utilize novel predictive markers of RBC quality identified by -omics and machine learning to usher in a new era of safer and precise transfusion medicine.
Keywords: preservation; red blood cells; transfusion.
Conflict of interest statement
U.A.G. and Case Western Reserve University have financial interests in Hemex Health Inc. and Xatek Inc. U.A.G., E.K., and Case Western Reserve University have financial interests in BioChip Labs Inc. U.A.G. has financial interests in DxNow Inc. Financial interests include licensed intellectual property, stock ownership, research funding, employment, and consulting. Hemex Health Inc. offers point-of-care diagnostics for hemoglobin disorders, anemia, and malaria. BioChip Labs Inc. offers commercial clinical microfluidic biomarker assays for inherited or acquired blood disorders. Xatek Inc. offers point-of-care global assays to evaluate the hemostatic process. DxNow Inc. offers microfluidic and bio-imaging technologies for in vitro fertilization, forensics, and diagnostics. Competing interests of Case Western Reserve University employees are overseen and managed by the Conflict of Interests Committee according to a Conflict-of-Interest Management Plan. The study was conducted prior to E.K. employment at IDEXX Laboratories, and the ideas expressed are not in his capacity as an IDEXX employee. A.D. serves on the Scientific Advisory Board of Hemanext Inc. and Macopharma Inc.; his competing interests are managed by the University of Colorado in accordance with their conflict-of-interest policies. A.D. owns stock in Omix Technologies Inc. A.D. has multiple patents on novel blood storage strategies. S.N.T., M.T., R.D.S., and M.L.Y. have multiple patent applications that relate blood storage and storage in general. The remaining authors declare no competing interest.
Figures


References
-
- Klein H. G., Spahn D. R., Carson J. L., Red blood cell transfusion in clinical practice. Lancet 370, 415–426 (2007). - PubMed
-
- World Health Organization, “Global status report on blood safety and availability 2021” (2022). https://www.who.int/publications/i/item/9789240051683.
-
- Moore S. B., A brief history of the early years of blood transfusion at the Mayo Clinic: The first blood bank in the United States (1935). Transfus. Med. Rev. 19, 241–245 (2005). - PubMed
-
- Stansbury L. G., Hess J. R., Blood transfusion in World War I: The roles of Lawrence Bruce Robertson and Oswald Hope Robertson in the “most important medical advance of the war”. Transfus. Med. Rev. 23, 232–236 (2009). - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL146442/HL/NHLBI NIH HHS/United States
- 1R21GM141683/GM/NIGMS NIH HHS/United States
- R21 GM140656/GM/NIGMS NIH HHS/United States
- R21 GM136002/GM/NIGMS NIH HHS/United States
- R01HL148151/HL/NHLBI NIH HHS/United States
- R21 GM141683/GM/NIGMS NIH HHS/United States
- R01 HL148151/HL/NHLBI NIH HHS/United States
- R21 HL150032/HL/NHLBI NIH HHS/United States
- R01 HL149714/HL/NHLBI NIH HHS/United States
- R01HL157803/HL/NHLBI NIH HHS/United States
- R21HL150032/HL/NHLBI NIH HHS/United States
- R01AR081529/AR/NIAMS NIH HHS/United States
- R00HL143149/HL/NHLBI NIH HHS/United States
- R01 HL157803/HL/NHLBI NIH HHS/United States
- R01HL146442/HL/NHLBI NIH HHS/United States
- 5R01HL145031/HL/NHLBI NIH HHS/United States
- R01 AR081529/AR/NIAMS NIH HHS/United States
- R01 DK134590/DK/NIDDK NIH HHS/United States
- R01HL149714/HL/NHLBI NIH HHS/United States
- 5R21GM136002/GM/NIGMS NIH HHS/United States
- R00 HL143149/HL/NHLBI NIH HHS/United States
- R01 HL145031/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

