Frequency, compliance, and yield of cardiac testing after high-sensitivity troponin accelerated diagnostic protocol implementation
- PMID: 37494772
- PMCID: PMC10616758
- DOI: 10.1016/j.ajem.2023.07.014
Frequency, compliance, and yield of cardiac testing after high-sensitivity troponin accelerated diagnostic protocol implementation
Abstract
Background: Among persons presenting to the emergency department with suspected acute myocardial infarction (MI), cardiac troponin (cTn) testing is commonly used to detect acute myocardial injury. Accelerated diagnostic protocols (ADPs) guide clinicians to integrate cTn results with other clinical information to decide whether to order further diagnostic testing.
Objective: To determine the change in the rate and yield of stress test or coronary CT angiogram following cTn measurement in patients with chest pain presenting to the emergency department pre- and post-transition to a high-sensitivity (hs-cTn) assay in an updated ADP.
Methods: Using electronic health records, we examined visits for chest pain at five emergency departments affiliated with an integrated academic health system 1-year pre- and post-hs-cTn assay transition. Outcomes included stress test or coronary imaging frequency, ADP compliance among those with additional testing, and diagnostic yield (ratio of positive tests to total tests).
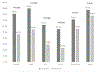
Results: There were 7564 patient-visits for chest pain, including 3665 in the pre- and 3899 in the post-period. Following the updated ADP using hs-cTn, 862 (23.5 per 100 patient visits) visits led to subsequent testing versus 1085 (27.8 per 100 patient visits) in the pre-hs-cTn period, (P < 0.001). Among those who were tested, the protocol-compliant rate fell from 80.9% to 46.5% (P < 0.001), but the yield of those tests rose from 24.5% to 29.2% (P = 0.07). Among tests that were noncompliant with ADP guidance, yield was similar pre- and post-updated hs-cTn ADP implementation (pre 13.0%, post 15.4% (P = 0.43).
Conclusion: Implementation of hs-cTn supported by an updated ADP was associated with a lower rate of stress testing and coronary CT angiogram.
Keywords: Accelerated diagnostic protocol; Acute myocardial infarction; Coronary CT angiogram; Emergency department; High-sensitivity troponin assay; Stress test.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest C.W.B. is a paid speaker for Roche Diagnostics and has previously participated in a Roche Advisory Board and is an advisor to Lucia Health Guidelines. I.G. reports consulting fees from F-Prime Capital. B.M.S. reports research grants via Brigham and Women's Hospital from AstraZeneca, Eisai, Novartis, and Merck and consulting fees from AstraZeneca, Biogen Idec, Boehringer Ingelheim, Covance, Dr. Reddy's Laboratory, Eisai, Elsevier Practice Update Cardiology, GlaxoSmithKline, Lexicon, Merck, NovoNordisk, Sanofi, St. Jude's Medical, and equity in Health [at] Scale. J.L.J. is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals and a Director at Jana Care; has received research support from Abbott, Applied Therapeutics, Innolife, Novartis Pharmaceuticals, and Roche Diagnostics; has received consulting income from Abbott, Beckman, Bristol Myers, Boehringer-Ingelheim, Janssen, Novartis, Pfizer, Merck, Roche Diagnostics and Siemens; and participates in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Bayer, CVRx, Intercept, Janssen, and Takeda. D.A.M. reports research grants to Brigham and Women's Hospital from Abbott Laboratories, Amgen, AstraZeneca/MedImmune, Daiichi Sankyo, Eisai, GlaxoSmithKline, Medicines Company, Merck, Novartis, Roche Diagnostics, and Takeda. He has received consulting fees from Abbott Laboratories, Aralez, AstraZeneca, Bayer, InCarda, Merck, Peloton, Roche Diagnostics, and Verseon. J.T.N.'s institution has received research funding on his behalf to perform research for Alere/Biosite/Quidel, Roche/IMARC, and Thermo-Fisher.
Figures
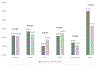
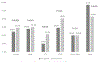

Similar articles
-
High-sensitivity cardiac troponin assays: From improved analytical performance to enhanced risk stratification.Crit Rev Clin Lab Sci. 2017 May;54(3):143-172. doi: 10.1080/10408363.2017.1285268. Epub 2017 May 1. Crit Rev Clin Lab Sci. 2017. PMID: 28457177 Review.
-
Impact of high-sensitivity cardiac troponin implementation on emergency department length of stay, testing, admissions, and diagnoses.Am J Emerg Med. 2021 Jul;45:54-60. doi: 10.1016/j.ajem.2021.02.021. Epub 2021 Feb 19. Am J Emerg Med. 2021. PMID: 33662739
-
Downstream Cascades of Care Following High-Sensitivity Troponin Test Implementation.J Am Coll Cardiol. 2021 Jun 29;77(25):3171-3179. doi: 10.1016/j.jacc.2021.04.049. Epub 2021 May 3. J Am Coll Cardiol. 2021. PMID: 34167642 Free PMC article.
-
Implementation of High-Sensitivity Cardiac Troponin Assays in the United States.J Am Coll Cardiol. 2023 Jan 24;81(3):207-219. doi: 10.1016/j.jacc.2022.10.017. Epub 2022 Oct 31. J Am Coll Cardiol. 2023. PMID: 36328155 Free PMC article.
-
High-Sensitivity Cardiac Troponin-Based Strategies for the Assessment of Chest Pain Patients-A Review of Validation and Clinical Implementation Studies.Clin Chem. 2018 Nov;64(11):1572-1585. doi: 10.1373/clinchem.2018.287342. Epub 2018 Jun 25. Clin Chem. 2018. PMID: 29941466 Review.
Cited by
-
Daily Heart Rate per Step: A Wearables Metric Associated With Cardiovascular Disease in a Cross-Sectional Study of the All of Us Research Program.J Am Heart Assoc. 2025 May 6;14(9):e036801. doi: 10.1161/JAHA.124.036801. Epub 2025 May 7. J Am Heart Assoc. 2025. PMID: 40156587 Free PMC article.
References
-
- Cairns CKK. National Hospital Ambulatory Medical Care Survey: 2019 emergency department summary tables. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2019-nhamcs-ed-web-table.... [accessed December 26 2022].
-
- Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of CHEST pain: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Cardiovasc Comput Tomogr. 2022;16(1):54–122. 10.1016/j.jcct.2021.11.009. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

