The 2023 wearable photoplethysmography roadmap
- PMID: 37494945
- PMCID: PMC10686289
- DOI: 10.1088/1361-6579/acead2
The 2023 wearable photoplethysmography roadmap
Abstract
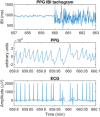
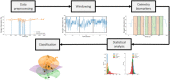
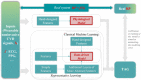
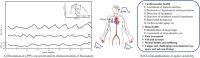
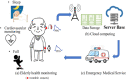
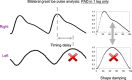
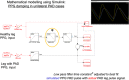
Photoplethysmography is a key sensing technology which is used in wearable devices such as smartwatches and fitness trackers. Currently, photoplethysmography sensors are used to monitor physiological parameters including heart rate and heart rhythm, and to track activities like sleep and exercise. Yet, wearable photoplethysmography has potential to provide much more information on health and wellbeing, which could inform clinical decision making. This Roadmap outlines directions for research and development to realise the full potential of wearable photoplethysmography. Experts discuss key topics within the areas of sensor design, signal processing, clinical applications, and research directions. Their perspectives provide valuable guidance to researchers developing wearable photoplethysmography technology.
Keywords: blood pressure; cardiovascular; fitness; physiological monitoring; sensor; signal processing; smartwatch.
Creative Commons Attribution license.
Conflict of interest statement
The authors declare there’s no conflict of interests. The views and opinions expressed are those of the authors and do not reflect those of the Ministry of Food and Drug Safety the in Republic of Korea.
The authors Jing Liu and Ni Zhao have a non-provisional patent (US10542894B2) for the multi-wavelength photoplethysmography-based techniques for cardiovascular monitoring.
Figures






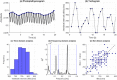
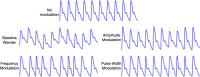
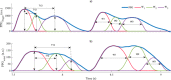





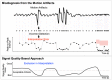





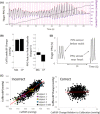
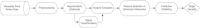
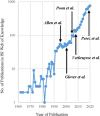
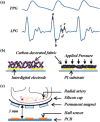
References
-
- Ajmal, Boonya-Ananta T, Rodriguez A J, Du Le V N, Ramella-Roman J C. Monte Carlo analysis of optical heart rate sensors in commercial wearables: the effect of skin tone and obesity on the photoplethysmography (PPG) signal. Biomed. Opt. Express. 2021;12:7445–57. doi: 10.1364/BOE.439893. - DOI - PMC - PubMed
-
- Ajtay B E, Béres S, Hejjel L. The oscillating pulse arrival time as a physiological explanation regarding the difference between ECG- and Photoplethysmogram-derived heart rate variability parameters. Biomed. Signal Process. Control. 2023;79:104033. doi: 10.1016/J.BSPC.2022.104033. - DOI
-
- Allen J. Photoplethysmography for the assessment of peripheral vascular disease. In: Kyriacou P A, Allen J, editors. Photoplethysmography: Technology, Signal Analysis and Applications. Elsevier; 2021. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
