Delayed-release rapamycin halts progression of left ventricular hypertrophy in subclinical feline hypertrophic cardiomyopathy: results of the RAPACAT trial
- PMID: 37495229
- PMCID: PMC10979416
- DOI: 10.2460/javma.23.04.0187
Delayed-release rapamycin halts progression of left ventricular hypertrophy in subclinical feline hypertrophic cardiomyopathy: results of the RAPACAT trial
Abstract
Objective: Feline hypertrophic cardiomyopathy (HCM) remains a disease with little therapeutic advancement. Rapamycin modulates the mTOR pathway, preventing and reversing cardiac hypertrophy in rodent disease models. Its use in human renal allograft patients is associated with reduced cardiac wall thickness. We sought to evaluate the effects of once-weekly delayed-release (DR) rapamycin over 6 months on echocardiographic, biochemical, and biomarker responses in cats with subclinical, nonobstructive HCM.
Animals: 43 client-owned cats with subclinical HCM.
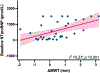
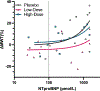
Methods: Cats enrolled in this double-blinded, multicentered, randomized, and placebo-controlled clinical trial were allocated to low- or high-dose DR rapamycin or placebo. Cats underwent physical examination, quality-of-life assessment, blood pressure, hematology, biochemistry, total T4, urinalysis, N-terminal pro-B-type natriuretic peptide, and cardiac troponin I at baseline and days 60, 120, and 180. Fructosamine was analyzed at screening and day 180. Echocardiograms were performed at all time points excluding day 120. Outcome variables were compared using a repeated measures ANCOVA.
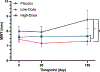
Results: No demographic, echocardiographic, or clinicopathologic values were significantly different between study groups at baseline, confirming successful randomization. At day 180, the primary study outcome variable, maximum LV myocardial wall thickness at any location, was significantly lower in the low-dose DR rapamycin group compared to placebo (P = .01). Oral DR rapamycin was well tolerated with no significant differences in adverse events between groups.
Clinical relevance: Results demonstrate that DR rapamycin was well tolerated and may prevent or delay progressive LV hypertrophy in cats with subclinical HCM. Additional studies are warranted to confirm and further characterize these results.
Keywords: HCM; cat; mTOR; occult; sirolimus.
Figures
References
-
- Fox PR, Keene BW, Lamb K, et al. International collaborative study to assess cardiovascular risk and evaluate long-term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: the REVEAL Study. J Vet Intern Med. 2018;32(3):930–943. doi:10.1111/jvim.15122 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

