Prognostic value of spinal cord lesion measures in early relapsing-remitting multiple sclerosis
- PMID: 37495267
- PMCID: PMC10804039
- DOI: 10.1136/jnnp-2023-331799
Prognostic value of spinal cord lesion measures in early relapsing-remitting multiple sclerosis
Abstract
Background: Spinal cord (SC) lesions have been associated with unfavourable clinical outcomes in multiple sclerosis (MS). However, the relation of whole SC lesion number (SCLN) and volume (SCLV) to the future occurrence and type of confirmed disability accumulation (CDA) remains largely unexplored.
Methods: In this monocentric retrospective study, SC lesions were manually delineated. Inclusion criteria were: age between 18 and 60 years, relapsing-remitting MS, disease duration under 2 years and clinical follow-up of 5 years. The first CDA event after baseline, determined by a sustained increase in the Expanded Disability Status Scale over 6 months, was classified as either progression independent of relapse activity (PIRA) or relapse-associated worsening (RAW). SCLN and SCLV were compared between different (sub)groups to assess their prospective value.
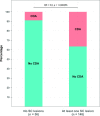
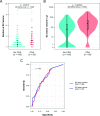
Results: 204 patients were included, 148 of which had at least one SC lesion and 59 experienced CDA. Patients without any SC lesions experienced significantly less CDA (OR 5.8, 95% CI 2.1 to 19.8). SCLN and SCLV were closely correlated (rs=0.91, p<0.001) and were both significantly associated with CDA on follow-up (p<0.001). Subgroup analyses confirmed this association for patients with PIRA on CDA (34 events, p<0.001 for both SC lesion measures) but not for RAW (25 events, p=0.077 and p=0.22).
Conclusion: Patients without any SC lesions are notably less likely to experience CDA. Both the number and volume of SC lesions on MRI are associated with future accumulation of disability largely independent of relapses.
Keywords: MRI; clinical neurology; image analysis; multiple sclerosis.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: VP has received research funding from Novartis (Oppenheim Förderpreis 2017). AB has received consulting and/or speaker fees from Alexion, Biogen, Celgene, Horizon, Novartis, Roche and Sandoz/Hexal. His institution has received compensation for clinical trials from Alexion, Biogen, Merck, Novartis, Roche, and Sanofi Genzyme. JSK has received research funding from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG; project 432290010), the German Federal Ministry of Education and Research (13GW0469D) and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (101045128—iBack-epic—ERC-2021-COG). He is Co-Founder of Bonescreen. BH has served on scientific advisory boards for Novartis; he has served as DMSC member for AllergyCare, Sandoz, Polpharma, Biocon and TG therapeutics; his institution received research grants from Roche for multiple sclerosis research. He has received honoraria for counseling (Gerson Lehrmann Group). He holds part of two patents; one for the detection of antibodies against KIR4.1 in a subpopulation of patients with multiple sclerosis and one for genetic determinants of neutralising antibodies to interferon.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
