Nitric oxide-induced ribosome collision activates ribosomal surveillance mechanisms
- PMID: 37495584
- PMCID: PMC10372077
- DOI: 10.1038/s41419-023-05997-5
Nitric oxide-induced ribosome collision activates ribosomal surveillance mechanisms
Abstract
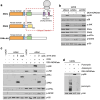
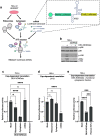
Impairment of protein translation can cause stalling and collision of ribosomes and is a signal for the activation of ribosomal surveillance and rescue pathways. Despite clear evidence that ribosome collision occurs stochastically at a cellular and organismal level, physiologically relevant sources of such aberrations are poorly understood. Here we show that a burst of the cellular signaling molecule nitric oxide (NO) reduces translational activity and causes ribosome collision in human cell lines. This is accompanied by activation of the ribotoxic stress response, resulting in ZAKα-mediated activation of p38 and JNK kinases. In addition, NO production is associated with ZNF598-mediated ubiquitination of the ribosomal protein RPS10 and GCN2-mediated activation of the integrated stress response, which are well-described responses to the collision of ribosomes. In sum, our work implicates a novel role of NO as an inducer of ribosome collision and activation of ribosomal surveillance mechanisms in human cells.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737. - PubMed
-
- Wagner EF, Nebreda ÁR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. - PubMed
-
- Nikolic I, Leiva M, Sabio G. The role of stress kinases in metabolic disease. Nat Rev Endocrinol. 2020;16:697–716. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

