Generation of whole tumor cell vaccine for on-demand manipulation of immune responses against cancer under near-infrared laser irradiation
- PMID: 37495590
- PMCID: PMC10372023
- DOI: 10.1038/s41467-023-40207-y
Generation of whole tumor cell vaccine for on-demand manipulation of immune responses against cancer under near-infrared laser irradiation
Abstract
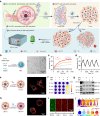
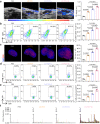
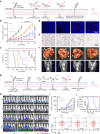
The therapeutic efficacy of whole tumor cell vaccines (TCVs) is modest, which has delayed their translation into personalized immunotherapies in the clinic. Here, we develop a TCV platform based on photothermal nanoparticle-loaded tumor cells, which can be rationally applied to diverse tumor types to achieve on-demand boost of anti-tumor immune responses for inhibiting tumor growth. During the fabrication process, mild photothermal heating by near-infrared (NIR) laser irradiation induces the nanoparticle-bearing tumor cells to express heat shock proteins as endogenous adjuvants. After a single vaccination at the back of tumor-bearing mice, non-invasive NIR laser irradiation further induces mild hyperthermia at vaccination site, which promotes the recruitment, activation, and antigen presentation by dendritic cells. Using an indicator we term fluctuation of tumor growth rate, we determine appropriate irradiation regimens (including optimized irradiation intervals and times). This TCV platform enables on-demand NIR manipulation of immune responses, and we demonstrate potent therapeutic efficacy against six murine models that mimick a range of clinical scenarios, including a model based on humanized mice and patient-derived tumor xenografts.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
An Endogenous Vaccine Based on Fluorophores and Multivalent Immunoadjuvants Regulates Tumor Micro-Environment for Synergistic Photothermal and Immunotherapy.Theranostics. 2018 Jan 1;8(3):860-873. doi: 10.7150/thno.19826. eCollection 2018. Theranostics. 2018. PMID: 29344312 Free PMC article.
-
From perinuclear to intranuclear localization: A cell-penetrating peptide modification strategy to modulate cancer cell migration under mild laser irradiation and improve photothermal therapeutic performance.Biomaterials. 2019 Dec;223:119443. doi: 10.1016/j.biomaterials.2019.119443. Epub 2019 Aug 23. Biomaterials. 2019. PMID: 31541779
-
Evaluation of a nanocomposite of PEG-curcumin-gold nanoparticles as a near-infrared photothermal agent: an in vitro and animal model investigation.Lasers Med Sci. 2018 Nov;33(8):1769-1779. doi: 10.1007/s10103-018-2538-1. Epub 2018 May 22. Lasers Med Sci. 2018. PMID: 29790012
-
IR792-MCN@ZIF-8-PD-L1 siRNA drug delivery system enhances photothermal immunotherapy for triple-negative breast cancer under near-infrared laser irradiation.J Nanobiotechnology. 2022 Mar 2;20(1):96. doi: 10.1186/s12951-022-01255-6. J Nanobiotechnology. 2022. PMID: 35236356 Free PMC article.
-
Near-infrared light triggered drug delivery system for higher efficacy of combined chemo-photothermal treatment.Acta Biomater. 2017 Mar 15;51:374-392. doi: 10.1016/j.actbio.2016.12.004. Epub 2017 Jan 11. Acta Biomater. 2017. PMID: 28088668
Cited by
-
NIR-II-activated whole-cell vaccine with ultra-efficient semiconducting diradical oligomers for breast carcinoma growth and metastasis inhibition.Acta Pharm Sin B. 2025 Feb;15(2):1159-1170. doi: 10.1016/j.apsb.2024.12.017. Epub 2024 Dec 20. Acta Pharm Sin B. 2025. PMID: 40177542 Free PMC article.
-
Allogeneic "Zombie Cell" as Off-The-Shelf Vaccine for Postsurgical Cancer Immunotherapy.Adv Sci (Weinh). 2024 Apr;11(13):e2307030. doi: 10.1002/advs.202307030. Epub 2024 Jan 26. Adv Sci (Weinh). 2024. PMID: 38279587 Free PMC article.
-
Tumor Vaccines for Malignant Melanoma: Progress, Challenges, and Future Directions.Oncol Res. 2025 Jul 18;33(8):1875-1893. doi: 10.32604/or.2025.063843. eCollection 2025. Oncol Res. 2025. PMID: 40746887 Free PMC article. Review.
-
Polymeric nanocarrier via metabolism regulation mediates immunogenic cell death with spatiotemporal orchestration for cancer immunotherapy.Nat Commun. 2024 Oct 4;15(1):8586. doi: 10.1038/s41467-024-53010-0. Nat Commun. 2024. PMID: 39362879 Free PMC article.
-
Engineering Challenges and Opportunities in Autologous Cellular Cancer Immunotherapy.J Immunol. 2024 Jan 15;212(2):188-198. doi: 10.4049/jimmunol.2300642. J Immunol. 2024. PMID: 38166251 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

