Rewiring cancer drivers to activate apoptosis
- PMID: 37495688
- PMCID: PMC10749586
- DOI: 10.1038/s41586-023-06348-2
Rewiring cancer drivers to activate apoptosis
Erratum in
-
Author Correction: Rewiring cancer drivers to activate apoptosis.Nature. 2023 Sep;621(7977):E27. doi: 10.1038/s41586-023-06543-1. Nature. 2023. PMID: 37596490 Free PMC article. No abstract available.
Abstract
Genes that drive the proliferation, survival, invasion and metastasis of malignant cells have been identified for many human cancers1-4. Independent studies have identified cell death pathways that eliminate cells for the good of the organism5,6. The coexistence of cell death pathways with driver mutations suggests that the cancer driver could be rewired to activate cell death using chemical inducers of proximity (CIPs). Here we describe a new class of molecules called transcriptional/epigenetic CIPs (TCIPs) that recruit the endogenous cancer driver, or a downstream transcription factor, to the promoters of cell death genes, thereby activating their expression. We focused on diffuse large B cell lymphoma, in which the transcription factor B cell lymphoma 6 (BCL6) is deregulated7. BCL6 binds to the promoters of cell death genes and epigenetically suppresses their expression8. We produced TCIPs by covalently linking small molecules that bind BCL6 to those that bind to transcriptional activators that contribute to the oncogenic program, such as BRD4. The most potent molecule, TCIP1, increases binding of BRD4 by 50% over genomic BCL6-binding sites to produce transcriptional elongation at pro-apoptotic target genes within 15 min, while reducing binding of BRD4 over enhancers by only 10%, reflecting a gain-of-function mechanism. TCIP1 kills diffuse large B cell lymphoma cell lines, including chemotherapy-resistant, TP53-mutant lines, at EC50 of 1-10 nM in 72 h and exhibits cell-specific and tissue-specific effects, capturing the combinatorial specificity inherent to transcription. The TCIP concept also has therapeutic applications in regulating the expression of genes for regenerative medicine and developmental disorders.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
G.R.C. is a founder and scientific advisor for Foghorn Therapeutics and Shenandoah Therapeutics. N.S.G. is a founder, science advisory board member (SAB) and equity holder in Syros, C4, Allorion, Lighthorse, Voronoi, Inception, Matchpoint, CobroVentures, GSK, Shenandoah (board member), Larkspur (board member) and Soltego (board member). The Gray laboratory receives or has received research funding from Novartis, Takeda, Astellas, Taiho, Jansen, Kinogen, Arbella, Deerfield, Springworks, Interline and Sanofi. T.Z. is a scientific founder, equity holder and consultant of Matchpoint, equity holder of Shenandoah, and consultant of Lighthorse. M.R.G. reports research funding from Sanofi, Kite/Gilead, Abbvie and Allogene; consulting for Abbvie, Allogene and Bristol Myers Squibb; honoraria from Tessa Therapeutics, Monte Rosa Therapeutics and Daiichi Sankyo; and stock ownership of KDAc Therapeutics. Shenandoah has a license from Stanford for the TCIP technology that was invented by G.R.C., S.G., A.K., C-Y.C, W.W., S.H.K., N.S.G., W.J., X.L. and Z.L. All other authors declare no competing interests.
Figures








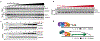




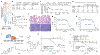
Comment in
-
Activating apoptosis in B cell lymphoma.Nat Rev Drug Discov. 2023 Sep;22(9):697. doi: 10.1038/d41573-023-00128-3. Nat Rev Drug Discov. 2023. PMID: 37532825 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

