Real-world treatment outcomes of medicines used in special situations (off-label and compassionate use) in oncology and hematology: A retrospective study from a comprehensive cancer institution
- PMID: 37496404
- PMCID: PMC10501253
- DOI: 10.1002/cam4.6360
Real-world treatment outcomes of medicines used in special situations (off-label and compassionate use) in oncology and hematology: A retrospective study from a comprehensive cancer institution
Abstract
Purpose: Medicines in special situations (MSS) refer to off-label or to unlicensed drugs under investigation (compassionate use). Our objectives were to evaluate characteristics and to estimate overall survival (OS), event-free survival (EFS), and the duration of treatment (DT) of MSS used for cancer treatment at a multicentre comprehensive cancer institution.
Methods: Retrospective cohort study on adult cancer patients for whom an MSS treatment was requested (January 2011-December 2020). A descriptive analysis was performed and median OS and EFS and 95% confidence intervals (CIs) were estimated. Survival curves were stratified by type of tumor, ECOG (Eastern Cooperative Oncology Group) performance status (PS), age, sex, treatment stage and type of drug (mechanism of action and target).
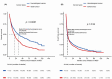
Results: Treatment was initiated in 2092 episodes (1930 patients) out of 2377 MSS episodes (2189 patients) requested, 33% for hematological treatment and 87% for advanced stage cancer. Median OS (months) was 21.1 (95% CI 19.4-22.7), median EFS was 5.6 (95% CI 5.1-6.0) months, and median DT was 4.5 [0.0; 115.3] months. OS and EFS statistically significantly favored female patients, ECOG PS ≥2 episodes showed worse OS and EFS outcomes (p < 0.0001). Statistically significant differences in survival were found within solid and hematological cancer, disease stage, drug mechanism of action, and type of cancer (p < 0.001) but not for age. Survival outcomes by tumor subtype and drug are presented both globally and separately based on disease stage.
Conclusion: MSS uses are practiced across almost all cancer types, mostly for advanced disease. ECOG PS ≥2, along with advanced disease, was related to worse survival. Information about real-world outcomes is valuable and contributes to better decision-making regarding MSS and our experience in this field could be of interest for other colleagues.
Keywords: cancer; compassionate use; off-label use; survival; treatment outcomes.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The author(s) declare(s) that there are no conflicts of interest regarding the publication of this article.
Figures
References
-
- 2021 FDA approvals of drugs for cancer treatment—the ASCO post . 2021. https://ascopost.com/issues/december‐25‐2021/2021‐fda‐approvals‐of‐drugs...
-
- Weda M, Hoebert J, Moltó Puigmarti C, et al. Study on off‐label use of medicinal products in the European Union. Eur Union. 2017;1‐185. https://www.nivel.nl/sites/default/files/bestanden/Report_OFF_LABEL_Nive...
-
- Lee VC. Off‐label drug information: regulation, distribution, evaluation, and related controversies. P&T. 2009;34(8):428‐40. https://pubmed.ncbi.nlm.nih.gov/20140107/ - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical