Loss of TRPC2 function in mice alters sex differences in brain regions regulating social behaviors
- PMID: 37496437
- PMCID: PMC10642801
- DOI: 10.1002/cne.25528
Loss of TRPC2 function in mice alters sex differences in brain regions regulating social behaviors
Abstract
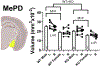
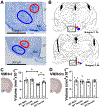
The transient receptor potential cation channel 2 (TRPC2) conveys pheromonal information from the vomeronasal organ (VNO) to the brain. Both male and female mice lacking this gene show altered sex-typical behavior as adults. We asked whether TRPC2, highly expressed in the VNO, normally participates in the development of VNO-recipient brain regions controlling mounting and aggression, two behaviors affected by TRPC2 loss. We now report significant effects of TRPC2 loss in both the posterodorsal aspect of the medial amygdala (MePD) and ventromedial nucleus of the hypothalamus (VMH) of male and female mice. In the MePD, a sex difference in neuron number was eliminated by the TRPC2 knockout (KO), but the effect was complex, with fewer neurons in the right MePD of females, and fewer neurons in the left MePD of males. In contrast, MePD astrocytes were unaffected by the KO. In the ventrolateral (vl) aspect of the VMH, KO females were like wildtype (WT) females, but TRPC2 loss had a dramatic effect in males, with fewer neurons than WT males and a smaller VMHvl overall. We also discovered a glial sex difference in VMHvl of WTs, with females having more astrocytes than males. Interestingly, TRPC2 loss increased astrocyte number in males in this region. We conclude that TRPC2 normally participates in the sexual differentiation of the mouse MePD and VMHvl. These changes in two key VNO-recipient regions may underlie the effects of the TRPC2 KO on behavior.
Keywords: amygdala; astrocyte; hypothalamus; laterality; neuron; sex difference; stereology.
© 2023 The Authors. The Journal of Comparative Neurology published by Wiley Periodicals LLC.
Conflict of interest statement
Conflict of interest: The authors claim no conflict of interest.
Figures
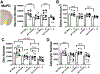

Similar articles
-
Sex and laterality differences in medial amygdala neurons and astrocytes of adult mice.J Comp Neurol. 2016 Aug 15;524(12):2492-502. doi: 10.1002/cne.23964. Epub 2016 Feb 3. J Comp Neurol. 2016. PMID: 26780286 Free PMC article.
-
Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala.J Comp Neurol. 2008 Dec 10;511(5):599-609. doi: 10.1002/cne.21859. J Comp Neurol. 2008. PMID: 18853427 Free PMC article.
-
Trpc2 gene impacts on maternal aggression, accessory olfactory bulb anatomy and brain activity.Genes Brain Behav. 2009 Oct;8(7):639-49. doi: 10.1111/j.1601-183X.2009.00511.x. Genes Brain Behav. 2009. PMID: 19799641 Free PMC article.
-
Neurobiology of TRPC2: from gene to behavior.Pflugers Arch. 2005 Oct;451(1):61-71. doi: 10.1007/s00424-005-1432-4. Epub 2005 Jun 22. Pflugers Arch. 2005. PMID: 15971083 Review.
-
The TRPC2 ion channel and pheromone sensing in the accessory olfactory system.Naunyn Schmiedebergs Arch Pharmacol. 2005 Apr;371(4):245-50. doi: 10.1007/s00210-005-1028-8. Naunyn Schmiedebergs Arch Pharmacol. 2005. PMID: 15871013 Review.
Cited by
-
Short and long duration testosterone treatments induce reversable subfertility in female mice using a gestational model of gender-affirming hormone therapy.Hum Reprod. 2025 Apr 1;40(4):695-706. doi: 10.1093/humrep/deaf016. Hum Reprod. 2025. PMID: 39935255
-
Neural basis for pheromone signal transduction in mice.Front Neural Circuits. 2024 Apr 29;18:1409994. doi: 10.3389/fncir.2024.1409994. eCollection 2024. Front Neural Circuits. 2024. PMID: 38742089 Free PMC article. Review.
References
-
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Hormones and behavior. 1985;19(4):469–98. - PubMed
-
- Kiselyov K, van Rossum DB, Patterson RL. TRPC channels in pheromone sensing. Vitam Horm. 2010;83:197–213. - PubMed
-
- Alekseyenko O, Baum M, Cherry J. Sex and gonadal steroid modulation of pheromone receptor gene expression in the mouse vomeronasal organ. Neuroscience. 2006;140(4):1349–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

