Carbapenem-Resistant Acinetobacter spp Infection in Critically Ill Patients With Limited Treatment Options: A Descriptive Study of Cefiderocol Therapy During the COVID-19 Pandemic
- PMID: 37496600
- PMCID: PMC10368198
- DOI: 10.1093/ofid/ofad329
Carbapenem-Resistant Acinetobacter spp Infection in Critically Ill Patients With Limited Treatment Options: A Descriptive Study of Cefiderocol Therapy During the COVID-19 Pandemic
Abstract
Background: Carbapenem-resistant Acinetobacter baumannii infections are difficult to treat and are a significant public health threat due to intrinsic/acquired resistance and limited treatment options.
Methods: A retrospective, observational cohort study in patients receiving cefiderocol via Shionogi's early access program for Acinetobacter spp infections (1 April 2020-30 April 2021; 27 sites; Italy, Spain, Germany, France). Primary outcome was clinical success, defined as clinical resolution of infection at day 14 or day 28 survival.
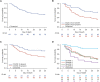
Results: Overall, 147 patients were included. Primary infection sites were respiratory (65.3%) and bloodstream (unknown source [15.6%]; catheter-related [10.9%]); 24.5% of patients had polymicrobial infection. Of 136 patients in intensive care (92.5%), 85.3% (116/136) received mechanical ventilation. Septic shock (55.6% [70/126]) and coronavirus disease 2019 (COVID-19) (81.6%) were prevalent. Prior to cefiderocol, 85.0% of patients received gram-negative treatment, 61.2% received ≥2 antimicrobials, and most received colistin (58.5%; median duration, 11.5 days). Cefiderocol monotherapy was used in 30.6% of patients. Clinical success rate was 53.1% and was higher in patients without septic shock (62.5%), without COVID-19 (77.8%), and with lower Sequential Organ Failure Assessment (SOFA) scores (quartile 1 [median, 3; range, 0-5]: 82.9%). Day 28 survival was 44.9% and was higher in patients without septic shock (60.7%), without COVID-19 (59.3%), with lower SOFA score (quartile 1: 82.9%), and receiving first-line cefiderocol (68.2% [15/22]). Resolution of infection at day 14 occurred in 39.5% of patients.
Conclusions: Despite use in complex patients with limited treatment options and high septic shock/COVID-19 rates, cefiderocol treatment was associated with an overall clinical success rate of 53%.
Keywords: Acinetobacter; COVID-19; cefiderocol; mortality; septic shock.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. M. G. has received honoraria for lectures, presentations, or speaker’s bureaus from MSD, Shionogi, Pfizer, and Gilead, and for participation on a data safety monitoring board or advisory board from MSD and Pfizer. S. V., A. K., and A. C. are employees of Shionogi B.V. H. D. has received fees from Shionogi for conferences and expert advisory boards. A. S. has received honoraria for lectures and advisory boards from Pfizer, MSD, Shionogi, Menarini, and Gilead. A. S. H. is an employee of Maxel Consulting ApS (Jyllinge, Denmark) and is a contractor for Shionogi B.V. M. F. has received grants and/or speaker honoraria from MSD, Angelini, Shionogi, Pfizer, Gilead, Menarini, and Nordic Pharma. H. A. H. reports no potential conflicts of interest.
Figures

References
-
- World Health Organization . Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis. 2017. Available at:https://apps.who.int/iris/handle/10665/311820. Accessed 6 June 2023.
-
- European Centre for Disease Prevention and Control . Rapid risk assessment: carbapenem-resistant Acinetobacter baumannii in healthcare settings. 2016. Available at:https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Pub.... Accessed 6 June 2023.
LinkOut - more resources
Full Text Sources
Miscellaneous

