The luminescence mechanism of ligand-induced interface states in silicon quantum dots
- PMID: 37496620
- PMCID: PMC10368006
- DOI: 10.1039/d3na00251a
The luminescence mechanism of ligand-induced interface states in silicon quantum dots
Abstract
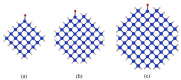
Over decades of research on photoluminescence (PL) of silicon quantum dots (Si-QDs), extensive exploratory experiments have been conducted to find ways to improve the photoluminescence quantum yield. However, the complete physical picture of Si-QD luminescence is not yet clear and needs to be studied in depth. In this work, which considers the quantum size effect and surface effect, the optical properties of Si-QDs with different sizes and surface terminated ligands were calculated based on first principles calculations. The results show that there are significant differences in the emission wavelength and emission intensity of Si-QD interface states connected by different ligands, among which the emission of silicon-oxygen double bonds is the strongest. When the size of the Si-QD increases, the influence of the surface effect weakens, and only the silicon-oxygen double bonds still localize the charge near the ligand, maintaining a high-intensity luminescence. In addition, the presence of surface dangling bonds also affects luminescence. This study deepens the understanding of the photoluminescence mechanism of Si-QDs, and provides a direction for both future improvement of the photoluminescence quantum efficiency of silicon nanocrystals and for fabricating silicon-based photonic devices.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
LinkOut - more resources
Full Text Sources

