Size-controlled liquid phase synthesis of colloidally stable Co3O4 nanoparticles
- PMID: 37496621
- PMCID: PMC10367999
- DOI: 10.1039/d3na00032j
Size-controlled liquid phase synthesis of colloidally stable Co3O4 nanoparticles
Abstract
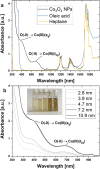
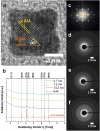
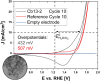
Spinel cobalt(ii,iii) oxide (Co3O4) represents a p-type semiconductor exhibiting promising functional properties in view of applications in a broad range of technological fields including magnetic materials and gas sensors as well as sustainable energy conversion systems based on photo- and electrocatalytic water splitting. Due to their high specific surface area, nanoparticle-based structures appear particularly promising for such applications. However, precise control over the diameter and the particle size distribution is required to achieve reproducible size-dependent properties. We herein introduce a synthetic strategy based on the decomposition of hydroxide precursors for the size-controlled preparation of purified Co3O4 nanoparticles with narrow size distributions adjustable in the range between 3-13 nm. The particles exhibit excellent colloidal stability. Their dispersibility in diverse organic solvents further facilitates processing (i.e. ligand exchange) and opens exciting perspectives for controlled self-assembly of the largely isometric primary particles into mesoscale structures. In view of potential applications, functional properties including absorption characteristics and electrocatalytic activity were probed by UV-Vis spectroscopy and cyclic voltammetry, respectively. In these experiments, low amounts of dispersed Co3O4 particles demonstrate strong light absorbance across the entire visible range and immobilized nanoparticles exhibit a comparably low overpotential towards the oxygen evolution reaction in electrocatalytic water splitting.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Dual-capable spinel cobalt oxide nanoparticles for electrocatalytic oxygen evolution and water contaminant removal.Environ Sci Pollut Res Int. 2024 Aug 15. doi: 10.1007/s11356-024-34682-z. Online ahead of print. Environ Sci Pollut Res Int. 2024. PMID: 39145907
-
Unravelling faradaic electrochemical efficiencies over Fe/Co spinel metal oxides using surface spectroscopy and microscopy techniques.Nanoscale. 2022 Nov 3;14(42):15928-15941. doi: 10.1039/d2nr04170g. Nanoscale. 2022. PMID: 36268905
-
Virus-directed formation of electrocatalytically active nanoparticle-based Co3O4 tubes.Nanoscale. 2017 May 18;9(19):6334-6345. doi: 10.1039/c7nr00508c. Nanoscale. 2017. PMID: 28387406
-
Alcohol Solvent Effects in the Synthesis of Co3O4 Metal-Oxide Nanoparticles: Disproof of a Surface-Ligand Thermodynamic Effect en Route to Alternative Kinetic and Thermodynamic Explanations.Inorg Chem. 2018 Feb 5;57(3):1517-1526. doi: 10.1021/acs.inorgchem.7b02831. Epub 2018 Jan 24. Inorg Chem. 2018. PMID: 29363962
-
Anchoring Co3O4 nanoparticles on MXene for efficient electrocatalytic oxygen evolution.Sci Bull (Beijing). 2020 Mar 30;65(6):460-466. doi: 10.1016/j.scib.2019.12.020. Epub 2019 Dec 28. Sci Bull (Beijing). 2020. PMID: 36747435
References
LinkOut - more resources
Full Text Sources

