Human neutrophils communicate remotely via calcium-dependent glutamate-induced glutamate release
- PMID: 37496680
- PMCID: PMC10366500
- DOI: 10.1016/j.isci.2023.107236
Human neutrophils communicate remotely via calcium-dependent glutamate-induced glutamate release
Abstract
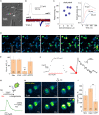
Neutrophils are white blood cells that are critical to acute inflammatory and adaptive immune responses. Their swarming-pattern behavior is controlled by multiple cellular cascades involving calcium-dependent release of various signaling molecules. Previous studies have reported that neutrophils express glutamate receptors and can release glutamate but evidence of direct neutrophil-neutrophil communication has been elusive. Here, we hold semi-suspended cultured human neutrophils in patch-clamp whole-cell mode to find that calcium mobilization induced by stimulating one neutrophil can trigger an N-methyl-D-aspartate (NMDA) receptor-driven membrane current and calcium signal in neighboring neutrophils. We employ an enzymatic-based imaging assay to image, in real time, glutamate release from neutrophils induced by glutamate released from their neighbors. These observations provide direct evidence for a positive-feedback inter-neutrophil communication that could contribute to mechanisms regulating communal neutrophil behavior.
Keywords: biological sciences; cell biology; immunology; molecular biology; neuroscience.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
NMDA receptor modulation of glutamate release in activated neutrophils.EBioMedicine. 2019 Sep;47:457-469. doi: 10.1016/j.ebiom.2019.08.004. Epub 2019 Aug 8. EBioMedicine. 2019. PMID: 31401196 Free PMC article.
-
Subanesthetic isoflurane relieves zymosan-induced neutrophil inflammatory response by targeting NMDA glutamate receptor and Toll-like receptor 2 signaling.Oncotarget. 2016 May 31;7(22):31772-89. doi: 10.18632/oncotarget.9091. Oncotarget. 2016. PMID: 27144523 Free PMC article.
-
Molecular regulation of neutrophil swarming in health and disease: Lessons from the phagocyte oxidase.iScience. 2023 Sep 26;26(10):108034. doi: 10.1016/j.isci.2023.108034. eCollection 2023 Oct 20. iScience. 2023. PMID: 37854699 Free PMC article. Review.
-
Human neutrophil Fc gamma receptors: different buttons for different responses.J Leukoc Biol. 2023 Nov 24;114(6):571-584. doi: 10.1093/jleuko/qiad080. J Leukoc Biol. 2023. PMID: 37437115 Review.
-
Spleen tyrosine kinase inhibitors disrupt human neutrophil swarming and antifungal functions.Microbiol Spectr. 2025 Jan 7;13(1):e0254921. doi: 10.1128/spectrum.02549-21. Epub 2024 Nov 27. Microbiol Spectr. 2025. PMID: 39601545 Free PMC article.
References
-
- Amulic B., Cazalet C., Hayes G.L., Metzler K.D., Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 2012;30:459–489. - PubMed
-
- Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. - PubMed
-
- Ng L.G., Ostuni R., Hidalgo A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 2019;19:255–265. - PubMed
-
- Häger M., Cowland J.B., Borregaard N. Neutrophil granules in health and disease. J. Intern. Med. 2010;268:25–34. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

