Active Ingredients from Chinese Medicine for Combination Cancer Therapy
- PMID: 37497002
- PMCID: PMC10367560
- DOI: 10.7150/ijbs.77720
Active Ingredients from Chinese Medicine for Combination Cancer Therapy
Abstract
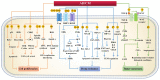
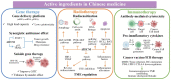
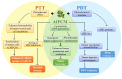
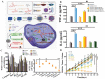
Combination therapy against cancer has gained increasing attention because it can help to target multiple pathways to tackle oncologic progression and improve the limited antitumor effect of single-agent therapy. Chinese medicine has been studied extensively in cancer therapy and proven to be efficacious in many cases due to its wide spectrum of anticancer activities. In this review, we aim to summarize the recent progress of active ingredients from Chinese medicine (AIFCM) in combination with various cancer therapeutic modalities, including chemotherapy, gene therapy, radiotherapy, phototherapy and immunotherapy. In addition to highlighting the potential contribution of AIFCM in combination cancer therapy, we also elucidate the underlying mechanisms behind their synergistic effect and improved anticancer efficacy, thereby encouraging the inclusion of these AIFCM as part of effective armamentarium in fighting intractable cancers. Finally, we present the challenges and future perspectives of AIFCM combination therapy as a feasible and promising strategy for the optimization of cancer treatment and better clinical outcomes.
Keywords: Active ingredients; Chinese medicine; Combination cancer therapy; Nanomedicine.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Huang W, Chen LQ, Kang L, Jin MJ, Sun P, Xin X. et al. Nanomedicine-based combination anticancer therapy between nucleic acids and small-molecular drugs. Adv Drug Deliv Rev. 2017;115:82–97. - PubMed
-
- Kemp JA, Shim MS, Heo CY, Kwon YJ. "Combo" nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv Drug Deliv Rev. 2016;98:3–18. - PubMed
-
- Huang L, Zhao SJ, Fang F, Xu T, Lan MH, Zhang JF. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials. 2021. 268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

