CO2 complexation with cyclodextrins
- PMID: 37497051
- PMCID: PMC10366446
- DOI: 10.3762/bjoc.19.78
CO2 complexation with cyclodextrins
Abstract
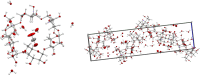
Carbon dioxide (CO2) emissions from industrial processes, power generation, and transportation contribute significantly to global warming and climate change. Carbon capture and storage (CCS) technologies are essential to reduce these emissions and mitigate the effects of climate change. Cyclodextrins (CDs), cyclic oligosaccharides, are studied as potential CO2 capture agents due to their unique molecular structures and high selectivity towards CO2. In this paper we have investigated binding efficiency of a number of cyclodextrins towards CO2. It is found that the crystal structure of α-cyclodextrin with CO2 has a 1:1 stoichioimetry and that a number of simple and modified cyclodextrins bind CO2 in water with a Kg of 0.18-1.2 bar-1 (7-35 M-1) with per-O-methyl α-cyclodextrin having the highest CO2 affinity.
Keywords: carbon dioxide; crystals; cyclodextrin; gas binding.
Copyright © 2023, Jessen et al.
Figures




References
-
- Keeling C D. Annu Rev Energy Environ. 1998;23:25–82. doi: 10.1146/annurev.energy.23.1.25. - DOI
-
- Evans J. Elements of a Sustainable World. Oxford University Press; 2020. - DOI
-
- Klaus S, Lehenmeier M W, Anderson C E, Rieger B. Coord Chem Rev. 2011;255:1460–1479. doi: 10.1016/j.ccr.2010.12.002. - DOI
-
- Ohno H, Ikhlayel M, Tamura M, Nakao K, Suzuki K, Morita K, Kato Y, Tomishige K, Fukushima Y. Green Chem. 2021;23:457–469. doi: 10.1039/d0gc03349a. - DOI
LinkOut - more resources
Full Text Sources
