Expression of SRP-9001 dystrophin and stabilization of motor function up to 2 years post-treatment with delandistrogene moxeparvovec gene therapy in individuals with Duchenne muscular dystrophy
- PMID: 37497476
- PMCID: PMC10366687
- DOI: 10.3389/fcell.2023.1167762
Expression of SRP-9001 dystrophin and stabilization of motor function up to 2 years post-treatment with delandistrogene moxeparvovec gene therapy in individuals with Duchenne muscular dystrophy
Abstract
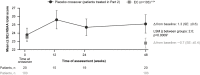
Introduction: Delandistrogene moxeparvovec (SRP-9001) is an investigational gene transfer therapy designed for targeted expression of SRP-9001 dystrophin protein, a shortened dystrophin retaining key functional domains of the wild-type protein. Methods: This Phase 2, double-blind, two-part (48 weeks per part) crossover study (SRP-9001-102 [Study 102]; NCT03769116) evaluated delandistrogene moxeparvovec in patients, aged ≥4 to <8 years with Duchenne muscular dystrophy. Primary endpoints (Part 1) were change from baseline (CFBL) in SRP-9001 dystrophin expression (Week 12), by Western blot, and in North Star Ambulatory Assessment (NSAA) score (Week 48). Safety assessments included treatment-related adverse events (TRAEs). Patients were randomized and stratified by age to placebo (n = 21) or delandistrogene moxeparvovec (n = 20) and crossed over for Part 2. Results: SRP-9001 dystrophin expression was achieved in all patients: mean CFBL to Week 12 was 23.82% and 39.64% normal in Parts 1 and 2, respectively. In Part 1, CFBL to Week 48 in NSAA score (least-squares mean, LSM [standard error]) was +1.7 (0.6) with treatment versus +0.9 (0.6) for placebo; p = 0.37. Disparity in baseline motor function between groups likely confounded these results. In 4- to 5-year-olds with matched baseline motor function, CFBL to Week 48 in NSAA scores was significantly different (+2.5 points; p = 0.0172), but not significantly different in 6-to-7-year-olds with imbalanced baseline motor function (-0.7 points; p = 0.5384). For patients treated with delandistrogene moxeparvovec in Part 2, CFBL to Week 48 in NSAA score was +1.3 (2.7), whereas for those treated in Part 1, NSAA scores were maintained. As all patients in Part 2 were exposed to treatment, results were compared with a propensity-score-weighted external control (EC) cohort. The LSM difference in NSAA score between the Part 2 treated group and EC cohort was statistically significant (+2.0 points; p = 0.0009). The most common TRAEs were vomiting, decreased appetite, and nausea. Most occurred within the first 90 days and all resolved. Discussion: Results indicate robust expression of SRP-9001 dystrophin and overall stabilization in NSAA up to 2 years post-treatment. Differences in NSAA between groups in Part 1 were not significant for the overall population, likely because cohorts were stratified only by age, and other critical prognostic factors were not well matched at baseline.
Keywords: AAVrh74; Duchenne muscular dystrophy; SRP-9001; dystrophin; gene therapy.
Copyright © 2023 Mendell, Shieh, McDonald, Sahenk, Lehman, Lowes, Reash, Iammarino, Alfano, Sabo, Woods, Skura, Mao, Staudt, Griffin, Lewis, Wang, Potter, Singh and Rodino-Klapac.
Conflict of interest statement
DG, SL, SW, RP, TS, LRR-K are empolyed by Sarepta Therapeutics Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Birnkrant D. J., Bushby K., Bann C. M., Apkon S. D., Blackwell A., Brumbaugh D., et al. (2018). Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 17, 251–267. 10.1016/S1474-4422(18)30024-3 - DOI - PMC - PubMed
-
- Clinicaltrials.gov (2012). NCT01603407: Finding the optimum regimen for Duchenne muscular dystrophy (FOR-DMD). Available: https://www.clinicaltrials.gov/ct2/show/NCT01603407 (Accessed February 3, 2023).
-
- Clinicaltrials.gov (2013). NCT01865084: A study of tadalafil for Duchenne muscular dystrophy. Available At: https://clinicaltrials.gov/ct2/show/NCT01865084 (Accessed February 3, 2023).
-
- Clinicaltrials.gov (2016). NCT00468832: Longitudinal study of the natural history of Duchenne muscular dystrophy (DMD). Available: https://clinicaltrials.gov/ct2/show/NCT00468832 (Accessed February 3, 2023).
LinkOut - more resources
Full Text Sources
Research Materials

