Visualization of surgical maneuvers using intraoperative real-time volumetric optical coherence tomography
- PMID: 37497507
- PMCID: PMC10368043
- DOI: 10.1364/BOE.488967
Visualization of surgical maneuvers using intraoperative real-time volumetric optical coherence tomography
Abstract
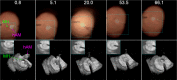
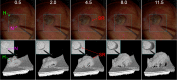
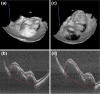
Ophthalmic microsurgery is traditionally performed using stereomicroscopes and requires visualization and manipulation of sub-millimeter tissue structures with limited contrast. Optical coherence tomography (OCT) is a non-invasive imaging modality that can provide high-resolution, depth-resolved cross sections, and has become a valuable tool in clinical practice in ophthalmology. While there has been substantial progress in both research and commercialization efforts to bring OCT imaging into live surgery, its use is still somewhat limited due to factors such as low imaging speed, limited scan configurations, and suboptimal data visualization. In this paper we describe, to the best of our knowledge, the translation of the fastest swept-source intraoperative OCT system with real-time volumetric imaging with stereoscopic data visualization provided via a heads-up display into the operating room. Results from a sampling of human anterior segment and retinal surgeries chosen from 93 human surgeries using the system are shown and the benefits that this mode of intrasurgical OCT imaging provides are discussed.
© 2023 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
AD: Duke University (P), CZ: Duke University (P), CAT: Duke University Medical Center (P), Alcon Laboratories (R), Theia Imaging (I,C), Emmes (C), LMV: Duke University Medical Center (P), Alcon Inc. (F,C), JAI: Duke University (P)
Figures



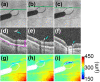
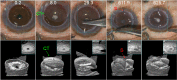

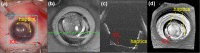
References
-
- Singh M., Saxena A., “Microsurgery: A Useful and Versatile Tool in Surgical Field,” Surgery: Curr. Res. 04(04), 4 (2014).10.4172/2161-1076.1000194 - DOI
-
- Izatt J. A., Hee M. R., Swanson E. A., Lin C. P., Huang D., Schuman J. S., Puliafito C. A., Fujimoto J. G., “Micrometer-Scale Resolution Imaging of the Anterior Eye In Vivo With Optical Coherence Tomography,” Arch. Ophthalmol. 112(12), 1584–1589 (1994).10.1001/archopht.1994.01090240090031 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
