Repetitive nociceptive stimulation elicits complex behavioral changes in Hirudo: evidence of arousal and motivational adaptations
- PMID: 37497630
- PMCID: PMC10445732
- DOI: 10.1242/jeb.245895
Repetitive nociceptive stimulation elicits complex behavioral changes in Hirudo: evidence of arousal and motivational adaptations
Abstract
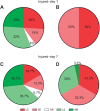
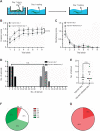
Appropriate responses to real or potential damaging stimuli to the body (nociception) are critical to an animal's short- and long-term survival. The initial goal of this study was to examine habituation of withdrawal reflexes (whole-body and local shortening) to repeated mechanical nociceptive stimuli (needle pokes) in the medicinal leech, Hirudo verbana, and assess whether injury altered habituation to these nociceptive stimuli. While repeated needle pokes did reduce shortening in H. verbana, a second set of behavior changes was observed. Specifically, animals began to evade subsequent stimuli by either hiding their posterior sucker underneath adjacent body segments or engaging in locomotion (crawling). Animals differed in terms of how quickly they adopted evasion behaviors during repeated stimulation, exhibiting a multi-modal distribution for early, intermediate and late evaders. Prior injury had a profound effect on this transition, decreasing the time frame in which animals began to carry out evasion and increasing the magnitude of these evasion behaviors (more locomotory evasion). The data indicate the presence in Hirudo of a complex and adaptive defensive arousal process to avoid noxious stimuli that is influenced by differences in internal states, prior experience with injury of the stimulated areas, and possibly learning-based processes.
Keywords: Arousal; Invertebrate; Leech; Nociception; Pain; Sensitization.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
Similar articles
-
Serotonin mediates stress-like effects on responses to non-nociceptive stimuli in the medicinal leech Hirudo verbana.J Exp Biol. 2022 Jun 1;225(11):jeb243404. doi: 10.1242/jeb.243404. Epub 2022 Jun 9. J Exp Biol. 2022. PMID: 35510636 Free PMC article.
-
Behavioral hierarchy in the medicinal leech, Hirudo medicinalis: feeding as a dominant behavior.Behav Brain Res. 1998 Jan;90(1):13-21. doi: 10.1016/s0166-4328(97)00072-7. Behav Brain Res. 1998. PMID: 9520210
-
Generalization of habituation and intrinsic sensitization in the leech.Learn Mem. 1998 Nov-Dec;5(6):405-19. Learn Mem. 1998. PMID: 10489258 Free PMC article.
-
[Hirudo medicinalis-leech applications in plastic and reconstructive microsurgery--a literature review].Handchir Mikrochir Plast Chir. 2007 Apr;39(2):103-7. doi: 10.1055/s-2007-965138. Handchir Mikrochir Plast Chir. 2007. PMID: 17497605 Review. German.
-
A classic model animal in the 21st century: recent lessons from the leech nervous system.J Exp Biol. 2015 Nov;218(Pt 21):3353-9. doi: 10.1242/jeb.113860. J Exp Biol. 2015. PMID: 26538172 Review.
Cited by
-
Injury alters sensory, motor, and integrative elements underlying operant conditioning in the medicinal leech.PLoS One. 2025 Jun 12;20(6):e0326039. doi: 10.1371/journal.pone.0326039. eCollection 2025. PLoS One. 2025. PMID: 40504817 Free PMC article.
References
-
- Appel, M. and Elwood, R. W. (2009). Motivational trade-offs and potential pain experience in hermit crabs. Appl. Anim. Behav. Sci. 119, 120-124. 10.1016/j.applanim.2009.03.013 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

