OPA1 deficiency impairs oxidative metabolism in cycling cells, underlining a translational approach for degenerative diseases
- PMID: 37497665
- PMCID: PMC10538295
- DOI: 10.1242/dmm.050266
OPA1 deficiency impairs oxidative metabolism in cycling cells, underlining a translational approach for degenerative diseases
Erratum in
-
Correction: OPA1 deficiency impairs oxidative metabolism in cycling cells, underlining a translational approach for degenerative diseases.Dis Model Mech. 2024 Apr 1;17(4):dmm050815. doi: 10.1242/dmm.050815. Epub 2024 Apr 26. Dis Model Mech. 2024. PMID: 38666499 Free PMC article. No abstract available.
Abstract
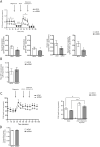
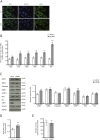
Dominant optic atrophy is an optic neuropathy with varying clinical symptoms and progression. A severe disorder is associated with certain OPA1 mutations and includes additional symptoms for >20% of patients. This underscores the consequences of OPA1 mutations in different cellular populations, not only retinal ganglionic cells. We assessed the effects of OPA1 loss of function on oxidative metabolism and antioxidant defences using an RNA-silencing strategy in a human epithelial cell line. We observed a decrease in the mitochondrial respiratory chain complexes, associated with a reduction in aconitase activity related to an increase in reactive oxygen species (ROS) production. In response, the NRF2 (also known as NFE2L2) transcription factor was translocated into the nucleus and upregulated SOD1 and GSTP1. This study highlights the effects of OPA1 deficiency on oxidative metabolism in replicative cells, as already shown in neurons. It underlines a translational process to use cycling cells to circumvent and describe oxidative metabolism. Moreover, it paves the way to predict the evolution of dominant optic atrophy using mathematical models that consider mitochondrial ROS production and their detoxifying pathways.
Keywords: Mathematical model; Mitochondria; Neurodegenerative disease; Oxidative metabolism.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
-
- Agier, V., Oliviero, P., Lainé, J., L'Hermitte-Stead, C., Girard, S., Fillaut, S., Jardel, C., Bouillaud, F., Bulteau, A. L. and Lombès, A. (2012). Defective mitochondrial fusion, altered respiratory function, and distorted cristae structure in skin fibroblasts with heterozygous OPA1 mutations. Biochim. Biophys. Acta 1822, 1570-1580. 10.1016/j.bbadis.2012.07.002 - DOI - PubMed
-
- Amati-Bonneau, P., Valentino, M. L., Reynier, P., Gallardo, M. E., Bornstein, B., Boissiere, A., Campos, Y., Rivera, H., de la Aleja, J. G., Carroccia, R.et al. (2008). OPA1 mutations induce mitochondrial DNA instability and optic atrophy ‘plus’ phenotypes. Brain 131, 338-351. 10.1093/brain/awm298 - DOI - PubMed
-
- Amati-Bonneau, P., Milea, D., Bonneau, D., Chevrollier, A., Ferré, M., Guillet, V., Gueguen, N., Loiseau, D., De Crescenzo, M.-A. P., Verny, C.et al. (2009). OPA1-associated disorders: phenotypes and pathophysiology. Int. J. Biochem. Cell Biol. 41, 1855-1865. 10.1016/j.biocel.2009.04.012 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

