Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation
- PMID: 37497794
- PMCID: PMC10374244
- DOI: 10.1002/14651858.CD003212.pub4
Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation
Abstract
Background: Nasal continuous positive airway pressure (NCPAP) is a useful method for providing respiratory support after extubation. Nasal intermittent positive pressure ventilation (NIPPV) can augment NCPAP by delivering ventilator breaths via nasal prongs.
Objectives: Primary objective To determine the effects of management with NIPPV versus NCPAP on the need for additional ventilatory support in preterm infants whose endotracheal tube was removed after a period of intermittent positive pressure ventilation. Secondary objectives To compare rates of abdominal distension, gastrointestinal perforation, necrotising enterocolitis, chronic lung disease, pulmonary air leak, mortality, duration of hospitalisation, rates of apnoea and neurodevelopmental status at 18 to 24 months for NIPPV and NCPAP. To compare the effect of NIPPV versus NCPAP delivered via ventilators versus bilevel devices, and assess the effects of the synchronisation of ventilation, and the strength of interventions in different economic settings.
Search methods: We used standard, extensive Cochrane search methods. The latest search date was January 2023.
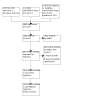
Selection criteria: We included randomised and quasi-randomised trials of ventilated preterm infants (less than 37 weeks' gestational age (GA)) ready for extubation to non-invasive respiratory support. Interventions were NIPPV and NCPAP.
Data collection and analysis: We used standard Cochrane methods. Our primary outcome was 1. respiratory failure. Our secondary outcomes were 2. endotracheal reintubation, 3. abdominal distension, 4. gastrointestinal perforation, 5. necrotising enterocolitis (NEC), 6. chronic lung disease, 7. pulmonary air leak, 8. mortality, 9. hospitalisation, 10. apnoea and bradycardia, and 11. neurodevelopmental status. We used GRADE to assess the certainty of evidence.
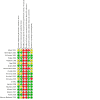
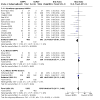
Main results: We included 19 trials (2738 infants). Compared to NCPAP, NIPPV likely reduces the risk of respiratory failure postextubation (risk ratio (RR) 0.75, 95% confidence interval (CI) 0.67 to 0.84; number needed to treat for an additional beneficial outcome (NNTB) 11, 95% CI 8 to 17; 19 trials, 2738 infants; moderate-certainty evidence) and endotracheal reintubation (RR 0.78, 95% CI 0.70 to 0.87; NNTB 12, 95% CI 9 to 25; 17 trials, 2608 infants, moderate-certainty evidence), and may reduce pulmonary air leaks (RR 0.57, 95% CI 0.37 to 0.87; NNTB 50, 95% CI 33 to infinite; 13 trials, 2404 infants; low-certainty evidence). NIPPV likely results in little to no difference in gastrointestinal perforation (RR 0.89, 95% CI 0.58 to 1.38; 8 trials, 1478 infants, low-certainty evidence), NEC (RR 0.86, 95% CI 0.65 to 1.15; 10 trials, 2069 infants; moderate-certainty evidence), chronic lung disease defined as oxygen requirement at 36 weeks (RR 0.93, 95% CI 0.84 to 1.05; 9 trials, 2001 infants; moderate-certainty evidence) and mortality prior to discharge (RR 0.81, 95% CI 0.61 to 1.07; 11 trials, 2258 infants; low-certainty evidence). When considering subgroup analysis, ventilator-generated NIPPV likely reduces respiratory failure postextubation (RR 0.49, 95% CI 0.40 to 0.62; 1057 infants; I2 = 47%; moderate-certainty evidence), while bilevel devices (RR 0.95, 95% CI 0.77 to 1.17; 716 infants) or a mix of both ventilator-generated and bilevel devices likely results in little to no difference (RR 0.87, 95% CI 0.73 to 1.02; 965 infants).
Authors' conclusions: NIPPV likely reduces the incidence of extubation failure and the need for reintubation within 48 hours to one-week postextubation more effectively than NCPAP in very preterm infants (GA 28 weeks and above). There is a paucity of data for infants less than 28 weeks' gestation. Pulmonary air leaks were also potentially reduced in the NIPPV group. However, it has no effect on other clinically relevant outcomes such as gastrointestinal perforation, NEC, chronic lung disease or mortality. Ventilator-generated NIPPV appears superior to bilevel devices in reducing the incidence of respiratory failure postextubation failure and need for reintubation. Synchronisation used to deliver NIPPV may be important; however, data are insufficient to support strong conclusions. Future trials should enrol a sufficient number of infants, particularly those less than 28 weeks' GA, to detect differences in death or chronic lung disease and should compare different categories of devices, establish the impact of synchronisation of NIPPV on safety and efficacy of the technique as well as the best combination of settings for NIPPV (rate, peak pressure and positive end-expiratory). Trials should strive to match the mean airway pressure between the intervention groups to allow a better comparison. Neurally adjusted ventilatory assist needs further assessment with properly powered randomised trials.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
BL was an author of an included study (Kirpalani 2013). The Canadian Institutes of Health Research funded the study. She requested data from the study for inclusion in the review (data extracted by the trial statistician, R Roberts). Roger Soll, co‐ordinating editor, Cochrane Neonatal assessed risk of bias and undertook GRADE assessment for this data. R Soll does not have any interests to disclose at this time.
PB: none.
MOD: none.
HK was involved in conducting a randomised controlled trial comparing NIPPV to NCPAP, (Nasal Ventilation in Preterm Infants (NIP) trial) published in the
AGP: none.
PD received a grant from the Australian National Health and Medical Research Council. He was involved in conducting the COIN trial (a multicentre trial conducted at his institution, The Royal Women's Hospital) by the Australian National Health and Medical Research Council. This trial is not included in this review.
Figures











































Update of
-
Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation.Cochrane Database Syst Rev. 2017 Feb 1;2(2):CD003212. doi: 10.1002/14651858.CD003212.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Jul 27;7:CD003212. doi: 10.1002/14651858.CD003212.pub4. PMID: 28146296 Free PMC article. Updated.
References
References to studies included in this review
Abyar 2011 {published data only}
-
- Abyar H, Ghafari V, Nakhshab M, Jafari M, Rahimi N, Asadpour S. Nasal intermittent mandatory ventilation (NIMV) versus nasal continuous positive airway pressure (NCPAP) in weaning from mechanical ventilation in preterm infants [مقایسه تاثیر دو روش تهویه متناوب از راه بینی )NIMV( و فشار مثبت مداوم راه هوایی از راه بینی )NCPAP( در قطع تنفس مصنوعی نوزادان]. Journal of Mazandaran University of Medical Sciences 2011;21(84):113-20. [URL: jmums.mazums.ac.ir/article-1-676-en.html]
Barrington 2001 {published and unpublished data}
-
- Barrington KJ, Bull D, Finer NN. Randomized controlled trial of nasal synchronized intermittent mandatory ventilation compared with continuous positive airway pressure after extubation of very low birth weight infants. Pediatrics 2001;107(4):638-41. [DOI: 10.1542/peds.107.4.638] [PMID: ] - DOI - PubMed
El‐Farrash 2022 {published data only}
Estay 2020 {published data only}
-
- Estay AS, Mariani GL, Alvarez CA, Milet B, Agost D, Avila CP, et al, for the NEOCOSUR Neonatal Network. Randomized controlled trial of nonsynchronized nasal intermittent positive pressure ventilation versus nasal CPAP after extubation of VLBW infants. Neonatology 2020;117(2):193-9. [DOI: 10.1159/000506164] [PMID: ] - DOI - PubMed
Friedlich 1999 {published data only}
-
- Friedlich P, Lecart C, Posen R, Ramicone E, Chan L, Ramanathan R. A randomized trial of nasopharyngeal-synchronised intermittent mandatory ventilation versus nasopharyngeal continuous positive airway pressure in very low birth weight infants following extubation. Journal of Perinatology 1999;19(6 Pt 1):413-8. [DOI: 10.1038/sj.jp.7200205] [PMID: ] - DOI - PubMed
Gao 2010 {published data only}
-
- Gao WW, Tan SZ, Chen YB, Zhang Y, Wang Y. Randomized trial of nasal synchronized intermittent mandatory ventilation compared with nasal continuous positive airway pressure in preterm infants with respiratory distress syndrome. Chinese Journal of Contemporary Pediatrics 2010;12(7):524-6. [PMID: ] - PubMed
Jasani 2016 {published data only}
-
- Jasani B, Nanavati R, Kabra N, Rajdeo S, Bhandari V. Comparison of non-synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as post-extubation respiratory support in preterm infants with respiratory distress syndrome: a randomized controlled trial. Journal of Maternal-Fetal & Neonatal Medicine 2016;29(10):1546-51. [DOI: 10.3109/14767058.2015.1059809] [PMID: ] - DOI - PubMed
Kahramaner 2014 {published data only}
-
- Kahramaner Z, Erdemir A, Turkoglu E, Cosar H, Sutcuoglu S, Ozer EA. Unsynchronized nasal intermittent positive pressure versus nasal continuous positive airway pressure in preterm infants after extubation. Journal of Maternal-Fetal & Neonatal Medicine 2014;27(9):926-9. [DOI: 10.3109/14767058.2013.846316] [PMID: ] - DOI - PubMed
Khalaf 2001 {published data only}
-
- Khalaf MN, Brodsky N, Hurley J, Bhandari V. A prospective randomized, controlled trial comparing synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as modes of extubation. Pediatrics 2001;108(1):13-7. [DOI: 10.1542/peds.108.1.13] [PMID: ] - DOI - PubMed
Khorana 2008 {published data only}
-
- Khorana M, Paradeevisut H, Sangtawesin V, Kanjanapatanakul W, Chotigeat U, Ayutthaya JK. A randomized trial of non-synchronized nasopharyngeal intermittent mandatory ventilation (nsNIMV) vs. nasal continuous positive airway pressure (nCPAP) in the prevention of extubation failure in preterm under 1500 grams. Journal of the Medical Association of Thailand 2008;91(3):S136-42. [PMID: ] - PubMed
Kirpalani 2013 {published and unpublished data}
Komatsu 2016 {published data only}
-
- Komatsu DF, Diniz EM, Ferraro AA, Ceccon ME, Vaz FA. Randomized controlled trial comparing nasal intermittent positive pressure ventilation and nasal continuous positive airway pressure in premature infants after tracheal extubation. Revista da Associação Médica Brasileira 2016;62(6):568-74. [DOI: 10.1590/1806-9282.62.06.568] [PMID: ] - DOI - PubMed
Li 2021 {published data only}
Moretti 2008 {published data only}
-
- Moretti C, Giannini L, Fassi C, Gizzi C, Papoff P, Colarizi P. Nasal flow-synchronized intermittent positive pressure ventilation to facilitate weaning in very low-birthweight infants: unmasked randomized controlled trial. Pediatrics International 2008;50(1):85-91. [DOI: 10.1111/j.1442-200X.2007.02525.x] [PMID: ] - DOI - PubMed
Najafian 2019 {published data only}
-
- Najafian B, Ansari-Benam I, Torkaman M, Khosravi MH. Comparing the efficacy of NCPAP and NIPPV in infants with RDS after extubation; a randomized clinical trial. Razavi International Journal of Medicine 2019;7(2):1-5. [DOI: 10.30483/RIJM.2019.118320] [PMID: ] - DOI
O'Brien 2012 {published data only}
-
- O'Brien K, Campbell C, Havlin L, Wenger L, Shah V. Infant flow biphasic nasal continuous positive airway pressure (BP-nCPAP) vs. infant flow nCPAP for the facilitation of extubation in infants less than or = 1250 grams: a randomized controlled trial. BMC Pediatrics 2012;12:43. [DOI: 10.1186/1471-2431-12-43] [PMID: ] - DOI - PMC - PubMed
Ribeiro 2017 {published data only}
-
- Ribeiro SN, Fontes MJ, Bhandari V, Resende CB, Johnston C. Noninvasive ventilation in newborns ≤ 1,500 g after tracheal extubation: randomized clinical trial. American Journal of Perinatology 2017;34(12):1190-8. Erratum in: American Journal of Perinatology 2017;34(12):e1-e2. [DOI: 10.1055/s-0037-1602141] [PMID: ] - DOI - PubMed
Victor 2016 {published data only}
Yllescas‐Medrano 2005 {published data only}
-
- Yllescas-Medrano E, Garcia MG, Martinez H, Guzman LA, Hernandez G, Cordero G, et al. Intermittent positive pressure using nasopharyngeal ventilation as a method to assist extubation among newborn infants less than 1500 grams [Ventilación nasofaríngea con presión positiva intermitente como método de extubación en recién nacidos pretérmino menores de 1,500 g]. Perinatology and Reproduction in Humans 2005;19(1):4-12. [scielo.org.mx/scielo.php?script=sci_arttext&pid=S0187-53372005000100...
References to studies excluded from this review
Aguiar 2015 {published data only}
-
- Aguiar T, Macedo I, Voutsen O, Silva P, Nona J, Araujo C, et al. Nasal bilevel versus continuous positive airway pressure in preterm infants: a randomized controlled trial. Journal of Clinical Trials 2015;5:3. [DOI: 10.4172/2167-0870.1000221] - DOI
Ali 2007 {published data only}
Armanian 2019 {published data only}
-
- Armanian AM, Iranpour R, Parvaneh M, Salehimehr N, Feizi A, Hajirezaei M. Heated humidified high flow nasal cannula (HHHFNC) is not an effective method for initial treatment of respiratory distress syndrome (RDS) versus nasal intermittent mandatory ventilation (NIMV) and nasal continuous positive airway pressure (NCPAP). Journal of Research in Medical Sciences 2019;24:73. [DOI: 10.4103/jrms.JRMS_2_19] [PMID: ] - DOI - PMC - PubMed
Bhandari 2007 {published data only}
Bisceglia 2007 {published data only}
-
- Bisceglia M, Belcastro A, Poerio V, Raimondi F, Mesuraca L, Crugliano C, et al. A comparison of nasal intermittent versus continuous positive pressure delivery for the treatment of moderate respiratory syndrome in preterm infants. Minerva Pediatrica 2007;59(2):91-5. [PMID: ] - PubMed
Bober 2012 {published data only}
-
- Bober K, Swietlinski J, Zejda J, Kornacka K, Pawlik D, Behrendt J, et al. A multicenter randomized controlled trial comparing effectiveness of two nasal continuous positive airway pressure devices in very-low-birth-weight infants. Pediatric Critical Care Medicine 2012;13(2):191. [DOI: 10.1097/PCC.0b013e3182231882] [PMID: ] - DOI - PubMed
Chen 2013 {published data only}
-
- Chen X, Peng WS, Wang L, Xu JL, Dong HF, Pan JH. A randomized controlled study of nasal intermittent positive pressure ventilation in the treatment of neonatal respiratory distress syndrome [经鼻间歇正压通气治疗新生儿呼吸窘迫综合征的随机对照研究]. Chinese Journal of Contemporary Pediatrics 2013;15(9):713-7. [PMID: ] - PubMed
DeSimone 2010 {published data only}
-
- DeSimone OA, Sommers R, Destin K, Mance M, Matook S, Stonestreet B, et al. Nasal intermittent positive pressure ventilation (NIPPV) does not facilitate earlier extubation in infants less than 28 weeks gestation: a pilot study. In: Pediatric Academic Societies (PAS) 2010 Annual Meeting; 2010 May 1-4; Vancouver, Canada. 2010. [CENTRAL: CN-00757316]
Dursun 2019 {published data only}
-
- Dursun M, Uslu S, Bulbul A, Celik M, Zubarioglu U, Bas EK. Comparison of early nasal intermittent positive pressure ventilation and nasal continuous positive airway pressure in preterm infants with respiratory distress syndrome. Journal of Tropical Pediatrics 2019;65(4):352-60. [DOI: 10.1093/tropej/fmy058] [PMID: ] - DOI - PubMed
Esmaeilnia 2016 {published data only}
Farhat 2018 {published data only}
-
- Farhat AS, Mohammadzadeh A, Mamuri GA, Saeidi R, Noorizadeh S. Comparison of nasal non-invasive ventilation methods in preterm neonates with respiratory distress syndrome. Iranian Journal of Neonatology 2018;9(4):53-60. [DOI: 10.22038/IJN.2018.24544.1313] - DOI
Fu 2014 {published data only}
-
- Fu CH, Xia SW. Clinical application of nasal intermittent positive pressure ventilation in initial treatment of neonatal respiratory distress syndrome [经鼻间歇正压通气在初始治疗早产儿呼吸窘迫综合征中的临床应用]. Chinese Journal of Contemporary Pediatrics 2014;16(5):460-4. [PMID: ] - PubMed
Gao 2014 {published data only}
-
- Gao X, Yang B, Hei M, Cui X, Wang J, Zhou G, et al. Application of three kinds of non-invasive positive pressure ventilation as a primary mode of ventilation in premature infants with respiratory distress syndrome: a randomized controlled trial. Chinese Journal of Pediatrics 2014;52(1):34-40. [PMID: ] - PubMed
Gharehbaghi 2019 {published data only}
-
- Gharehbaghi MM, Hosseini MB, Eivazi G, Yasrebinia S. Comparing the efficacy of nasal continuous positive airway pressure and nasal intermittent positive pressure ventilation in early management of respiratory distress syndrome in preterm infants. Oman Medical Journal 2019;34(2):99-104. [DOI: 10.5001/omj.2019.20] [PMID: ] - DOI - PMC - PubMed
Gomez 2018 {published data only}
-
- Gomez AK, Weerasekara M, Wickramaarachchi P, Prathapasinghe K, Wickramanayaka AW. Comparison of continuous positive airway pressure and non-invasive positive pressure ventilation as modes of non-invasive respiratory support for neonates in a Level III neonatal intensive care unit. Sri Lanka Journal of Child Health 2018;47(3):242-8. [DOI: 10.4038/sljch.v47i3.8547] - DOI
Kishore 2009 {published data only}
Kugelman 2007 {published data only}
-
- Kugelman A, Feferkorn I, Riskin A, Chistyakov I, Kaufman B, Bader D. Nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure for respiratory distress syndrome: a randomized controlled prospective study. Journal of Pediatrics 2007;150(5):521-6. [DOI: 10.1016/j.jpeds.2007.01.032] [PMID: ] - DOI - PubMed
Kumar 2011 {published data only}
Latremouille 2018 {published data only}
-
- Latremouille S, Al-Jabri A, Lamer P, Kanbar L, Shalish W, Kearney RE, et al. Heart rate variability in extremely preterm infants receiving nasal CPAP and non-synchronized noninvasive ventilation immediately after extubation. Respiratory Care 2018;63(1):62-9. [DOI: 10.4187/respcare.05672] [PMID: ] - DOI - PubMed
Latremouille 2021 {published data only}
Lin 1998 {published and unpublished data}
Manjunatha 2019 {published data only}
-
- Manjunatha CM, Kalyanasundaram S, Ibhanesebhor SE, Vigni D, Robertson C. Prospective randomized controlled trial comparing the use of biphasic positive airway pressure (BiPAP) with nasal continuous positive airway pressure (n-CPAP) following extubation of preterm babies. EC Paediatrics 2019;8(6):525-2.
Meneses 2011 {published data only}
Moretti 1999 {published data only}
-
- Moretti C, Gizzi C, Papoff P, Lampariello S, Capoferri M, Calcagnini G, et al. Comparing the effects of nasal synchronized intermittent positive pressure ventilation (nSIPPV) and nasal continuous positive airway pressure (nCPAP) after extubation in very low birth weight infants. Early Human Development 1999;56(2-3):166-77. [DOI: 10.1016/s0378-3782(99)00046-8] [PMID: ] - DOI - PubMed
Mukerji 2017 {published data only}
-
- Mukerji A, Sarmiento K, Lee B, Hassall K, Shah V. Non-invasive high-frequency ventilation versus bi-phasic continuous positive airway pressure (BP-CPAP) following CPAP failure in infants <1250g: a pilot randomized controlled trial. Journal of Perinatology 2017;37(1):49-53. [DOI: 10.1038/jp.2016.172] [PMID: ] - DOI - PubMed
Öktem 2021 {published data only}
Oncel 2016 {published data only}
-
- Oncel MY, Arayici S, Uras N, Alyamac-Dizdar E, Sari FN, Karahan S, et al. Nasal continuous positive airway pressure versus nasal intermittent positive-pressure ventilation within the minimally invasive surfactant therapy approach in preterm infants: a randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2016;101(4):F323-8. [DOI: 10.1136/archdischild-2015-308204] [PMID: ] - DOI - PubMed
Pan 2021 {published data only}
-
- Pan R, Chen GY, Wang J, Zhou ZX, Zhang PY, Chang LW, et al. Bi-level nasal positive airway pressure (BiPAP) versus nasal continuous positive airway pressure (CPAP) for preterm infants with birth weight less than 1500 g and respiratory distress syndrome following INSURE treatment: a two-center randomized controlled. Current Medical Science 2021;41(3):542-7. [DOI: 10.1007/s11596-021-2372-8] [PMID: ] - DOI - PMC - PubMed
Pantalitschka 2009 {published data only}
-
- Pantalitschka T, Sievers J, Urschitz MS, Herberts T, Reher C, Poets CF. Randomised crossover trial of four nasal respiratory support systems for apnoea of prematurity in very low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(4):F245-8. [DOI: 10.1136/adc.2008.148981] [PMID: ] - DOI - PubMed
Ramanathan 2012 {published data only}
-
- Ramanathan R, Sekar KC, Rasmussen M, Bhatia J, Soll RF. Nasal intermittent positive pressure ventilation after surfactant treatment for respiratory distress syndrome in preterm infants under 30 weeks gestation: a randomized, controlled trial. Journal of Perinatology 2012;32(5):336-43. [DOI: 10.1038/jp.2012.1g] [PMID: ] - DOI - PubMed
Ryan 1989 {published data only}
-
- Ryan CA, Finer NN, Peters KL. Nasal intermittent positive-pressure ventilation offers no advantages over nasal continuous positive airway pressure in apnea of prematurity. American Journal of Diseases in Childhood 1989;143(10):1196-8. [DOI: 10.1001/archpedi.1989.02150220094026] [PMID: ] - DOI - PubMed
Sabzehei 2018 {published data only}
-
- Sabzehei MK, Basiri B, Shokouhi M, Naser M. A comparative study of treatment response of respiratory distress syndrome in preterm infants: early nasal intermittent positive pressure ventilation versus early nasal continuous positive airway pressure. International Journal of Pediatrics 2018;6(10):8339-46. [DOI: 10.22038/ijp.2018.31577.2795] - DOI
Shi 2010 {published data only}
-
- Shi Y, Tang S, Zhao J, Hu Z, Li T. Efficiency of nasal intermittent positive pressure ventilation vs nasal continuous positive airway pressure on neonatal respiratory distress syndrome: a prospective, randomized, controlled study. Acta Academiae Medicinae Militaris Tertiae 2010;32(18):1991-4.
Shi 2013 {published data only}
Silveira 2015 {published data only}
Skariah 2019 {published data only}
-
- Skariah TA, Lewis LE. Early nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for respiratory distress syndrome (RDS) in infants of 28–36 weeks gestational age: a randomized controlled trial. Iranian Journal of Neonatology 2019;10(2):1-8. [DOI: 10.22038/IJN.2018.32566.1454] - DOI
Uchiyama 2020 {published data only}
-
- Uchiyama A, Okazaki K, Kondo M, Oka S, Motojima Y, Namba F, et al, Non-Invasive Procedure for Premature Neonates (NIPPN) Study Group. Randomized controlled trial of high-flow nasal cannula in preterm infants after extubation. Pediatrics 2020;146(6):e20201101. [DOI: 10.1542/peds.2020-1101] [PMID: ] - DOI - PubMed
Vieira 2021 {published data only}
-
- Vieira BD, Anchieta LM, Cardoso DR, Ribeiro SN, Ribeiro-Samora GA, Parreira VF. Effects of two modalities of noninvasive ventilation on breathing pattern of very low birth weight preterm infants immediately after extubation: a quasi-experimental study. Journal of Maternal-Fetal & Neonatal Medicine 2021;35(25):5717-23. [DOI: 10.1080/14767058.2021.1892063] [PMID: ] - DOI - PubMed
Yuan 2021 {published data only}
-
- Yuan G, Liu H, Wu Z, Chen X. Comparison of the efficacy and safety of three non-invasive ventilation methods in the initial treatment of premature infants with respiratory distress syndrome. International Journal of Clinical and Experimental Medicine 2021;14(2):1065-76. [ISSN:1940-5901/IJCEM0116814]
References to studies awaiting assessment
Ding 2020 {published data only}
Li 2022 {published data only}
Mostovoy 2012 {published data only}
-
- Mostovoy A, Romanenko K, Gorelik Y, Alexandrovich Y, Romanenko V, Gorelik K, et al. Use be-level nasal CPAP with variable flow in preterm infants after extubation: a multicenter randomized clinical study. Journal of Maternal-Fetal and Neonatal Medicine 2012;25(Suppl 2):1-115.
Saeedi 2012 {published data only}
-
- Saeedi R, Rahmani S, Norizade S. Effect of nasal synchronized intermittent mandatory ventilation versus nasal continuous positive airway pressure in reducing reintubation of extubated preterm infants. Archives of Disease in Childhood 2012;97(Suppl 2):A503-4. [DOI: 10.1136/archdischild-2012-302724.1780] - DOI
Yuan 2022 {published data only}
Zhu 2022 {published data only}
-
- Zhu X, Qi H, Feng Z, Shi Y, De Luca D, Nasal Oscillation Post-Extubation Study Group. Noninvasive high-frequency oscillatory ventilation vs nasal continuous positive airway pressure vs nasal intermittent positive pressure ventilation as postextubation support for preterm neonates in China: a randomized clinical trial. JAMA Pediatrics 2022;176(6):551-9. [DOI: 10.1001/jamapediatrics.2022.0710] [PMID: ] - DOI - PMC - PubMed
References to ongoing studies
Shi 2019 {published data only}
-
- NCT03181958. A trial comparing noninvasive ventilation strategies in preterm infants following extubation. clinicaltrials.gov/ct2/show/NCT03181958 (first received 9 June 2017).
-
- Shi Y, De Luca D, NASal OscillatioN post-Extubation (NASONE) study group. Continuous positive airway pressure (CPAP) vs noninvasive positive pressure ventilation (NIPPV) vs noninvasive high frequency oscillation ventilation (NHFOV) as post- extubation support in preterm neonates: protocol for an assessor-blinded, multicenter, randomized controlled trial. BMC Pediatrics 2019;19(1):256. [DOI: 10.1186/s12887-019-1625-1] [PMID: ] - DOI - PMC - PubMed
Additional references
Bancalari 2013
Cracknell 2023
-
- Cracknell J, Fiander M, McGuire W, Soll R, Bruschettini M, Mitra S. Template for Intervention review (as supplied January 2023). Cochrane Neonatal.
Davis 2003
Davis 2009
-
- Davis PG, Morley CJ, Owen LS. Non-invasive respiratory support of preterm neonates with respiratory distress: continuous positive airway pressure and nasal intermittent positive pressure ventilation. Seminars in Fetal and Neonatal Medicine 2009;14(1):14-20. [DOI: 10.1016/j.siny.2008.08.003] [PMID: ] - DOI - PubMed
de Medeiros 2012
Garland 1985
-
- Garland JS, Nelson DB, Rice T, Neu J. Increased risk of gastrointestinal perforations in neonates mechanically ventilated with either face mask or nasal prongs. Pediatrics 1985;76(3):406-10. [PMID: ] - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 1 June 2022. Hamilton (ON): McMaster University (developed by Evidence Prime), 2008. Available at gradepro.org.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2022
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Kiciman 1998
-
- Kiciman NM, Andréasson B, Bernstein G, Mannino FL, Rich W, Henderson C, et al. Thoracoabdominal motion in newborns during ventilation delivered by endotracheal tube or nasal prongs. Pediatric Pulmonology 1998;25(3):175-81. [DOI: 10.1002/(sici)1099-0496(199803)25:3<175::aid-ppul7>3.0.co;2-l] [PMID: ] - DOI - PubMed
Lee 2015
-
- Lee J, Kim HS, Jung YH, Shin SH, Choi CW, Kim EK, et al. Non-invasive neurally adjusted ventilatory assist in preterm infants: a randomised phase II crossover trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015;100(6):F507-13. [DOI: 10.1136/archdischild-2014-308057] [PMID: ] - DOI - PubMed
Lee 2019
Lemyre 2023
-
- Lemyre B, Deguise MO, Benson P, Kirpalani H, Ekhaguere OA, Davis PG. Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (nCPAP) for preterm infants. Cochrane Database of Systematic Reviews 2023, Issue 7. Art. No: CD005384. [DOI: 10.1002/14651858.CD005384.pub3] - DOI - PMC - PubMed
Liu 2022
-
- Liu H, Feng H, Zhang Y, Zhang L. Efficacy and safety of nasal intermittent positive pressure ventilation and nasal continuous positive airway pressure ventilation in neonatal respiratory distress syndrome: a systematic review and meta-analysis. Translational Pediatrics 2022;11(7):1242-50. [DOI: 10.21037/tp-22-288] [PMID: ] - DOI - PMC - PubMed
Moher 2009
Owen 2007
Owen 2008
Owen 2016
RevMan Web 2022 [Computer program]
-
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rüegger 2021
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shi 2019
-
- Shi Y, De Luca D, NASal OscillatioN post-Extubation (NASONE) study group. Continuous positive airway pressure (CPAP) vs noninvasive positive pressure ventilation (NIPPV) vs noninvasive high frequency oscillation ventilation (NHFOV) as post-extubation support in preterm neonates: protocol for an assessor-blinded, multicenter, randomized controlled trial. BMC Pediatrics 2019;19(1):256. [DOI: 10.1186/s12887-019-1625-1] [PMID: ] - DOI - PMC - PubMed
Solevåg 2021
Stein 2012
References to other published versions of this review
Davis 2001
-
- Davis PG, Lemyre B, De Paoli AG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2001, Issue 3. Art. No: CD003212. [DOI: 10.1002/14651858.CD003212] - DOI - PubMed
Lemyre 2014
-
- Lemyre B, Davis PG, De Paoli AG, Kirpalani H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD003212. [DOI: 10.1002/14651858.CD003212.pub2] - DOI - PubMed
Lemyre 2017
-
- Lemyre B, Davis PG, De Paoli AG, Kirpalani H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD003212. [DOI: 10.1002/14651858.CD003212.pub3] - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

