Characterization, Directed Evolution, and Targeting of DNA Virus-Encoded RNA Capping Enzymes Using Phenotypic Yeast Platforms
- PMID: 37498174
- PMCID: PMC11024868
- DOI: 10.1021/acschembio.3c00243
Characterization, Directed Evolution, and Targeting of DNA Virus-Encoded RNA Capping Enzymes Using Phenotypic Yeast Platforms
Abstract
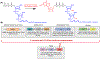
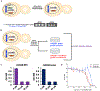
The constant and the sudden emergence of zoonotic human and animal viruses is a significant threat to human health, the world economy, and the world food supply. This has necessitated the development of broad-spectrum therapeutic strategies to combat these emerging pathogens. Mechanisms that are essential for viral replication and propagation have been successfully targeted in the past to develop broad-spectrum therapeutics that can be readily repurposed to combat new zoonotic pathogens. Because of the importance of viral RNA capping enzymes to viral replication and pathogenesis, as well as their presence in both DNA and RNA viruses, these viral proteins have been a long-standing therapeutic target. Here, we use genome sequencing information and yeast-based platforms (YeRC0M) to identify, characterize, and target viral genome-encoded essential RNA capping enzymes from emerging strains of DNA viruses, i.e., Monkeypox virus and African Swine Fever Virus, which are a significant threat to human and domestic animal health. We first identified and biochemically characterized these viral RNA capping enzymes and their necessary protein domains. We observed significant differences in functional protein domains and organization for RNA capping enzymes from emerging DNA viruses in comparison to emerging RNA viruses. We also observed several differences in the biochemical properties of these viral RNA capping enzymes using our phenotypic yeast-based approaches (YeRC0M) as compared to the previous in vitro studies. Further, using directed evolution, we were able to identify inactivation and attenuation mutations in these essential viral RNA capping enzymes; these data could have implications on virus biocontainment as well as live attenuated vaccine development. We also developed methods that would facilitate high-throughput phenotypic screening to identify broad-spectrum inhibitors that selectively target viral RNA capping enzymes over host RNA capping enzymes. As demonstrated here, our approaches to identify, characterize, and target viral genome-encoded essential RNA capping enzymes are highly modular and can be readily adapted for targeting emerging viral pathogens as well as their variants that emerge in the future.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures





References
-
- Florescu DF; Keck MA Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev. Anti-Infect. Ther 2014, 12, 1171–1178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

