Enhancement of Ammonium Oxidation at Microoxic Bioanodes
- PMID: 37498945
- PMCID: PMC10413939
- DOI: 10.1021/acs.est.3c02227
Enhancement of Ammonium Oxidation at Microoxic Bioanodes
Abstract
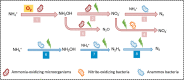
Bioelectrochemical systems (BESs) are considered to be energy-efficient to convert ammonium, which is present in wastewater. The application of BESs as a technology to treat wastewater on an industrial scale is hindered by the slow removal rate and lack of understanding of the underlying ammonium conversion pathways. This study shows ammonium oxidation rates up to 228 ± 0.4 g-N m-3 d-1 under microoxic conditions (dissolved oxygen at 0.02-0.2 mg-O2/L), which is a significant improvement compared to anoxic conditions (120 ± 21 g-N m-3 d-1). We found that this enhancement was related to the formation of hydroxylamine (NH2OH), which is rate limiting in ammonium oxidation by ammonia-oxidizing microorganisms. NH2OH was intermediate in both the absence and presence of oxygen. The dominant end-product of ammonium oxidation was dinitrogen gas, with about 75% conversion efficiency in the presence of a microoxic level of dissolved oxygen and 100% conversion efficiency in the absence of oxygen. This work elucidates the dominant pathways under microoxic and anoxic conditions which is a step toward the application of BESs for ammonium removal in wastewater treatment.
Keywords: Ammonium; ammonia-oxidizing microorganisms; bioanode; electro-anammox; oxygen.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
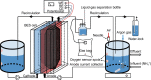
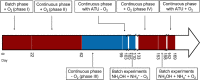

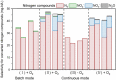

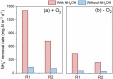
References
-
- Directorate-General for Environment , In Urban Waste Water Directive 98/15/EEC; European Commission: 1998. https://eur-lex.europa.eu/eli/dir/1998/15/oj (accessed 2023-07-17).
-
- Li Y.-h.; Li H.-b.; Xu X.-y.; Xiao S.-y.; Wang S.-q.; Xu S.-c. Fate of nitrogen in subsurface infiltration system for treating secondary effluent. Water Sci. Eng. 2017, 10 (3), 217–224. 10.1016/j.wse.2017.10.002. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

