The application of an isotropic crushable foam model to predict the femoral fracture risk
- PMID: 37498946
- PMCID: PMC10374151
- DOI: 10.1371/journal.pone.0288776
The application of an isotropic crushable foam model to predict the femoral fracture risk
Abstract
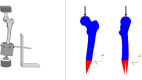
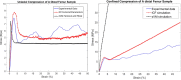
For biomechanical simulations of orthopaedic interventions, it is imperative to implement a material model that can realistically reproduce the nonlinear behavior of the bone structure. However, a proper material model that adequately combines the trabecular and cortical bone response is not yet widely identified. The current paper aims to investigate the possibility of using an isotropic crushable foam (ICF) model dependent on local bone mineral density (BMD) for simulating the femoral fracture risk. The elastoplastic properties of fifty-nine human femoral trabecular cadaveric bone samples were determined and combined with existing cortical bone properties to characterize two forms of the ICF model, a continuous and discontinuous model. Subsequently, the appropriateness of this combined material model was evaluated by simulating femoral fracture experiments, and a comparison with earlier published results of a softening Von-Mises (sVM) material model was made. The obtained mechanical properties of the trabecular bone specimens were comparable to previous findings. Furthermore, the ultimate failure load predicted by the simulations of femoral fractures was on average 79% and 90% for the continuous and discontinuous forms of the ICF model and 82% of the experimental value for the sVM material model. Also, the fracture locations predicted by ICF models were comparable to the experiments. In conclusion, a nonlinear material model dependent on BMD was characterized for human femoral bone. Our findings indicate that the ICF model could predict the femoral bone strength and reproduce the variable fracture locations in the experiments.
Copyright: © 2023 Soltanihafshejani et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

