Acyloxyacyl hydrolase promotes pulmonary defense by preventing alveolar macrophage tolerance
- PMID: 37498977
- PMCID: PMC10409266
- DOI: 10.1371/journal.ppat.1011556
Acyloxyacyl hydrolase promotes pulmonary defense by preventing alveolar macrophage tolerance
Abstract
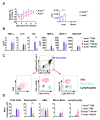
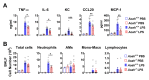
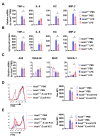
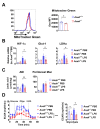
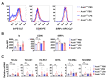
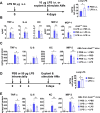
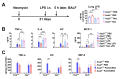
Although alveolar macrophages (AMs) play important roles in preventing and eliminating pulmonary infections, little is known about their regulation in healthy animals. Since exposure to LPS often renders cells hyporesponsive to subsequent LPS exposures ("tolerant"), we tested the hypothesis that LPS produced in the intestine reaches the lungs and stimulates AMs, rendering them tolerant. We found that resting AMs were more likely to be tolerant in mice lacking acyloxyacyl hydrolase (AOAH), the host lipase that degrades and inactivates LPS; isolated Aoah-/- AMs were less responsive to LPS stimulation and less phagocytic than were Aoah+/+ AMs. Upon innate stimulation in the airways, Aoah-/- mice had reduced epithelium- and macrophage-derived chemokine/cytokine production. Aoah-/- mice also developed greater and more prolonged loss of body weight and higher bacterial burdens after pulmonary challenge with Pseudomonas aeruginosa than did wildtype mice. We also found that bloodborne or intrarectally-administered LPS desensitized ("tolerized") AMs while antimicrobial drug treatment that reduced intestinal commensal Gram-negative bacterial abundance largely restored the innate responsiveness of Aoah-/- AMs. Confirming the role of LPS stimulation, the absence of TLR4 prevented Aoah-/- AM tolerance. We conclude that commensal LPSs may stimulate and desensitize (tolerize) alveolar macrophages in a TLR4-dependent manner and compromise pulmonary immunity. By inactivating LPS in the intestine, AOAH promotes antibacterial host defenses in the lung.
Copyright: © 2023 Cheng et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Ballinger MN, Paine R 3rd, Serezani CH, Aronoff DM, Choi ES, Standiford TJ, et al. Role of granulocyte macrophage colony-stimulating factor during gram-negative lung infection with Pseudomonas aeruginosa. American journal of respiratory cell and molecular biology. 2006;34(6):766–74. doi: 10.1165/rcmb.2005-0246OC ; PubMed Central PMCID: PMC2644237. - DOI - PMC - PubMed
-
- Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R 3rd, et al. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infection and immunity. 1997;65(4):1139–46. doi: 10.1128/iai.65.4.1139-1146.1997 ; PubMed Central PMCID: PMC175109. - DOI - PMC - PubMed
-
- Pittet LA, Quinton LJ, Yamamoto K, Robson BE, Ferrari JD, Algul H, et al. Earliest innate immune responses require macrophage RelA during pneumococcal pneumonia. American journal of respiratory cell and molecular biology. 2011;45(3):573–81. doi: 10.1165/rcmb.2010-0210OC ; PubMed Central PMCID: PMC3175578. - DOI - PMC - PubMed
-
- Schneider C, Nobs SP, Heer AK, Kurrer M, Klinke G, van Rooijen N, et al. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS pathogens. 2014;10(4):e1004053. doi: 10.1371/journal.ppat.1004053 ; PubMed Central PMCID: PMC3974877. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

