Treatment of strabismus and blepharospasm with Botox (onabotulinumtoxinA): Development, insights, and impact
- PMID: 37499080
- PMCID: PMC10374181
- DOI: 10.1097/MD.0000000000032374
Treatment of strabismus and blepharospasm with Botox (onabotulinumtoxinA): Development, insights, and impact
Abstract
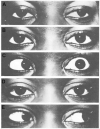
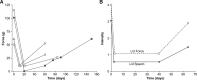
Strabismus, deviation of the ocular alignment, can adversely affect quality of life and activities of daily living. Surgery was the prior standard of care for strabismus, but up to 40% of patients required additional surgeries. This need for more effective and less invasive treatment, along with the convergence of other events such as the development of electromyography, purification of botulinum toxin A, and the finding that injection of botulinum toxin type A could paralyze the hind limbs of chicks, led Dr. Alan Scott to investigate injection of his formulation for strabismus. The positive results of initial trials in monkeys segued to human trials with observations of alignment improvements and few adverse events. The success of botulinum toxin type A in the treatment of strabismus led to interest in its use to treat other skeletal muscles, particularly in blepharospasm, a type of focal dystonia involving eyelid spasms and involuntary eye closure that lacked an effective pharmacological treatment. Patient groups helped to increase awareness of this novel treatment, and results from clinical trials confirmed its effectiveness. Dr. Scott's formulation, then known as Oculinum, received its first Food and Drug Administration approvals in 1989 for strabismus and blepharospasm. Allergan acquired Oculinum in 1991, renaming it Botox. These initial uses led to its application in a myriad of other indications as outlined in other articles of this supplement.
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc.
Figures



References
-
- Rutstein RP, Cogen MS, Cotter SA, et al. Optometric clinical practice guideline: care of the patient with strabismus: esotropia and exotropia. 2010. Available at: https://www.aoa.org/documents/optometrists/CPG-12.pdf.
-
- Hashemi H, Pakzad R, Heydarian S, et al. Global and regional prevalence of strabismus: a comprehensive systematic review and meta-analysis. Strabismus. 2019;27:54–65. - PubMed
-
- Hultman O, Beth Høeg T, Munch IC, et al. The Danish Rural Eye Study: prevalence of strabismus among 3785 Danish adults – a population-based cross-sectional study. Acta Ophthalmol. 2019;97:784–92. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

