Treatment of hyperhidrosis with Botox (onabotulinumtoxinA): Development, insights, and impact
- PMID: 37499084
- PMCID: PMC10374185
- DOI: 10.1097/MD.0000000000032764
Treatment of hyperhidrosis with Botox (onabotulinumtoxinA): Development, insights, and impact
Abstract
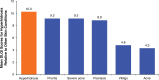
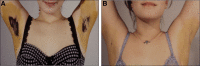
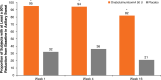
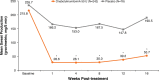
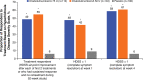
Hyperhidrosis (chronic excessive sweating) may substantially affect an individual's emotional and social well-being. Therapies available before onabotulinumtoxinA were generally topical, with limited effectiveness, application-site skin reactions, and frequent, time-consuming treatments. Intradermal injection of onabotulinumtoxinA to treat sweat glands arose as a novel therapeutic approach. To develop this treatment, appropriate dosing needed to be established, and training on administration was required. Further, no previous scale existed to measure the effects of hyperhidrosis on patients' lives, leading Allergan to develop and validate the 4-point Hyperhidrosis Disease Severity Scale (HDSS), which measures the disease's impact on daily activities. The onabotulinumtoxinA clinical development program for hyperhidrosis included 2 double-blind, placebo-controlled pivotal trials, immunogenicity studies, long-term studies of safety and efficacy, and quality of life assessments. In Europe and North America, the primary efficacy measures were, respectively, axillary sweat production measured gravimetrically and HDSS improvement. Compared with placebo, onabotulinumtoxinA treatment significantly reduced axillary sweat production and axillary hyperhidrosis severity, as measured by a 2-point or greater reduction on the HDSS. The effects of onabotulinumtoxinA occurred rapidly, within 1 week after injection, and lasted ≥6 months. Treatment with onabotulinumtoxinA was associated with significant quality of life improvements based on Short Form-12 physical and mental component scores. The Hyperhidrosis Impact Questionnaire also indicated greater treatment satisfaction, reduced negative impact on aspects of daily life, and improved emotional well-being with onabotulinumtoxinA versus placebo. The clinical development program and subsequent clinical experience showed that onabotulinumtoxinA treatment for hyperhidrosis was well tolerated with no new safety signals, and led to greater disease awareness.
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
This manuscript was funded by AbbVie. AbbVie was involved in the manuscript concept and participated in writing, reviewing, and approval of the final version. No honoraria or payments were made for authorship. N Lowe has received past consultant fees, honoraria, and research grants from Allergan (prior to its acquisition by AbbVie). He owns stock in AbbVie. M Naumann has no funding and conflicts of interest to disclose. N Eadie was an employee of Allergan Aesthetics, an AbbVie Company, at the time of this research. Writing and editorial assistance was provided to the authors by Michelle McDermott, PharmD of Peloton Advantage, LLC, an OPEN Health company, and was funded by AbbVie.
Figures


References
-
- Lessa Lda R, Luz FB, De Rezende RM, et al. . The psychiatric facet of hyperhidrosis: demographics, disability, quality of life, and associated psychopathology. J Psychiatr Pract. 2014;20:316–23. - PubMed
-
- Skroza N, Bernardini N, La Torre G, et al. . Correlation between dermatology life quality index and minor test and differences in their levels over time in patients with axillary hyperhidrosis treated with botulinum toxin type A. Acta Dermatovenerol Croat. 2011;19:16–20. - PubMed
-
- Weber A, Heger S, Sinkgraven R, et al. . Psychosocial aspects of patients with focal hyperhidrosis. Marked reduction of social phobia, anxiety and depression and increased quality of life after treatment with botulinum toxin A. Br J Dermatol. 2005;152:342–5. - PubMed
-
- Swartling C, Naver H, Lindberg M. Botulinum A toxin improves life quality in severe primary focal hyperhidrosis. Eur J Neurol. 2001;8:247–52. - PubMed
-
- Naumann MK, Hamm H, Lowe NJ. Effect of botulinum toxin type A on quality of life measures in patients with excessive axillary sweating: a randomized controlled trial. Br J Dermatol. 2002;147:1218–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

