Treatment of chronic migraine with Botox (onabotulinumtoxinA): Development, insights, and impact
- PMID: 37499085
- PMCID: PMC10374186
- DOI: 10.1097/MD.0000000000032600
Treatment of chronic migraine with Botox (onabotulinumtoxinA): Development, insights, and impact
Abstract
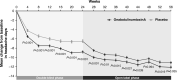
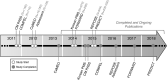
Chronic migraine (CM) is a neurological disease characterized by frequent migraine attacks that prevent affected individuals from performing daily activities of living, significantly diminish quality of life, and increase familial burden. Before onabotulinumtoxinA was approved for CM, there were few treatment options for these seriously disabled patients and none had regulatory approval. The terminology and recognition of CM evolved in parallel with the onabotulinumtoxinA clinical development program. Because there were no globally accepted classification criteria for CM when onabotulinumtoxinA was in development, the patient populations for the trials conducted by Allergan were determined by the Allergan migraine team in collaboration with headache scientists and clinicians. These trials and collaborations ultimately led to improvements in CM classifications. In 2010, onabotulinumtoxinA became the first medication and first biologic approved specifically to prevent headaches in patients with CM. Approval was based on 2 similarly designed phase 3, double-blind, randomized, placebo-controlled, multicenter clinical studies. Both studies showed significantly greater improvements in mean change from baseline in headache-day frequency in patients with CM receiving onabotulinumtoxinA compared with those receiving placebo. The safety and effectiveness of onabotulinumtoxinA have been established globally in >5000 patients with CM with or without medication overuse treated in clinical and observational studies. Benefits also include improvements in quality of life, fewer psychiatric comorbidities, and reduced healthcare resource utilization. Across studies, onabotulinumtoxinA was well tolerated; adverse events tended to be mild or moderate in severity and to decline over subsequent treatment cycles.
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
This study was funded by AbbVie. AbbVie was involved in the manuscript concept and participated in writing, reviewing, and approval of the final version. No honoraria or payments were made for authorship. MF Brin is a full-time employee of AbbVie and holds stock in the company.
Figures


References
-
- Manack A, Turkel CC, Kaplowitz H. Role of epidemiological data within the drug development lifecycle: a chronic migraine case study In: Lunet N, ed. Epidemiology – current perspectives on research and practice. Rijeka, Croatia: InTech. 2012:155–70.
-
- Natoli JL, Manack A, Dean B, et al. . Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010;30:599–609. - PubMed
-
- Grosberg B, Bigal ME, Ricci J, et al. . Epidemiology of chronic daily headache in adolescents [abstract]. Neurology. 2007;68(Suppl 1):A180.
-
- Whitcup SM, Turkel CC, DeGryse RE, et al. . Development of onabotulinumtoxinA for chronic migraine. Ann N Y Acad Sci. 2014;1329:67–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

