A human papillomavirus whole genome plasmid repository: A resource for HPV DNA quality control reagents
- PMID: 37499306
- PMCID: PMC10527912
- DOI: 10.1016/j.jcv.2023.105548
A human papillomavirus whole genome plasmid repository: A resource for HPV DNA quality control reagents
Abstract
Well characterized reference reagents are useful for assay validation, proficiency/competency assessment, daily run controls, and to improve inter-laboratory comparisons. Synthetic human papillomavirus (HPV) DNA fragments and plasmid clones are available, but synthetic fragments include limited segments of the HPV genome and many HPV plasmids have interrupted coding regions or contain partial genomes. As a result, they are not compatible with all HPV DNA detection and typing assays. To address this need, we are establishing an HPV plasmid repository of HPV clones containing the whole genome of each type with no interruptions in coding regions. To date, HPV plasmid clones for 16 HPV types, (including all vaccine types and 14 types in clinical assays: HPV6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) have been constructed using a Gibson assembly method and validated by sequencing and the Novaplex HPV typing assay. The newly constructed HPV whole genome plasmids can serve as a quality control reagent resource for HPV DNA assays and are available for public health and research laboratories.
Keywords: HPV typing; Human papillomavirus; Quality control reagents; Whole genome plasmid.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

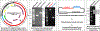

References
-
- Poljak M, Ostrbenk Valencak A, Gimpelj Domjanic G, Xu L, Arbyn M. Commercially available molecular tests for human papillomaviruses: a global overview. Clin Microbiol Infect 2020;26:1144–50. - PubMed
-
- Tota JE, Bentley J, Blake J, Coutlée F, Duggan MA, Ferenczy A, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: Acting on evidence to change the current paradigm. Preventive Medicine 2017;98:5–14. - PubMed
-
- Wilkinson DE, Baylis SA, Padley D, Heath AB, Ferguson M, Pagliusi SR, et al. Establishment of the 1st World Health Organization international standards for human papillomavirus type 16 DNA and type 18 DNA. Int J Cancer 2010;126:2969–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

