Optical coherence tomography angiography in diabetic retinopathy
- PMID: 37499857
- PMCID: PMC11268430
- DOI: 10.1016/j.preteyeres.2023.101206
Optical coherence tomography angiography in diabetic retinopathy
Abstract
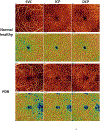
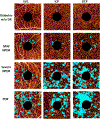
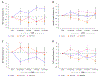
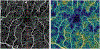
There remain many unanswered questions on how to assess and treat the pathology and complications that arise from diabetic retinopathy (DR). Optical coherence tomography angiography (OCTA) is a novel and non-invasive three-dimensional imaging method that can visualize capillaries in all retinal layers. Numerous studies have confirmed that OCTA can identify early evidence of microvascular changes and provide quantitative assessment of the extent of diseases such as DR and its complications. A number of informative OCTA metrics could be used to assess DR in clinical trials, including measurements of the foveal avascular zone (FAZ; area, acircularity, 3D para-FAZ vessel density), vessel density, extrafoveal avascular zones, and neovascularization. Assessing patients with DR using a full-retinal slab OCTA image can limit segmentation errors and confounding factors such as those related to center-involved diabetic macular edema. Given emerging data suggesting the importance of the peripheral retinal vasculature in assessing and predicting DR progression, wide-field OCTA imaging should also be used. Finally, the use of automated methods and algorithms for OCTA image analysis, such as those that can distinguish between areas of true and false signals, reconstruct images, and produce quantitative metrics, such as FAZ area, will greatly improve the efficiency and standardization of results between studies. Most importantly, clinical trial protocols should account for the relatively high frequency of poor-quality data related to sub-optimal imaging conditions in DR and should incorporate time for assessing OCTA image quality and re-imaging patients where necessary.
Keywords: Diabetic macular edema; Diabetic macular ischemia; Diabetic retinopathy; Optical coherence tomography; Optical coherence tomography angiography.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures






References
-
- Agemy SA, Scripsema NK, Shah CM, Chui T, Garcia PM, Lee JG, Gentile RC, Hsiao YS, Zhou Q, Ko T, Rosen RB, 2015. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina 35, 2353–2363. - PubMed
-
- Al-Sheikh M, Falavarjani KG, Tepelus TC, Sadda SR, 2017. Quantitative comparison of swept-source and spectral-domain OCT angiography in healthy eyes. Ophthalmic Surg. Lasers Imaging Retina 48, 385–391. - PubMed
-
- Alibhai AY, De Pretto LR, Moult EM, Or C, Arya M, McGowan M, Carrasco-Zevallos O, Lee B, Chen S, Baumal CR, Witkin AJ, Reichel E, de Freitas AZ, Duker JS, Fujimoto JG, Waheed NK, 2020. Quantification of retinal capillary nonperfusion in diabetics using wide-field optical coherence tomography angiography. Retina 40, 412–420. - PubMed

