A small molecule inhibitor of PTP1B and PTPN2 enhances T cell anti-tumor immunity
- PMID: 37500611
- PMCID: PMC10374545
- DOI: 10.1038/s41467-023-40170-8
A small molecule inhibitor of PTP1B and PTPN2 enhances T cell anti-tumor immunity
Abstract
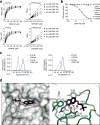
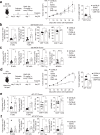
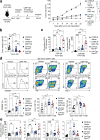
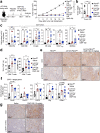
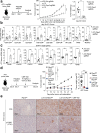
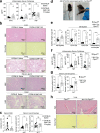
The inhibition of protein tyrosine phosphatases 1B (PTP1B) and N2 (PTPN2) has emerged as an exciting approach for bolstering T cell anti-tumor immunity. ABBV-CLS-484 is a PTP1B/PTPN2 inhibitor in clinical trials for solid tumors. Here we have explored the therapeutic potential of a related small-molecule-inhibitor, Compound-182. We demonstrate that Compound-182 is a highly potent and selective active site competitive inhibitor of PTP1B and PTPN2 that enhances T cell recruitment and activation and represses the growth of tumors in mice, without promoting overt immune-related toxicities. The enhanced anti-tumor immunity in immunogenic tumors can be ascribed to the inhibition of PTP1B/PTPN2 in T cells, whereas in cold tumors, Compound-182 elicited direct effects on both tumor cells and T cells. Importantly, treatment with Compound-182 rendered otherwise resistant tumors sensitive to α-PD-1 therapy. Our findings establish the potential for small molecule inhibitors of PTP1B and PTPN2 to enhance anti-tumor immunity and combat cancer.
© 2023. The Author(s).
Conflict of interest statement
F.W. and T.T. are inventors on pending patents related to this work filed by Monash University and the Peter MacCallum Cancer Centre. T.T. is on the scientific advisory board of DepYmed. Z.Y.Z. is a co-founder and serves on the scientific advisory board of Tyligand Bioscience. N.D.H. is a founder and shareholder in oNKo-Innate. N.D.H. serves on an advisory board for Bristol Myers Squibb. The remaining authors declare no competing interests.
Figures




Update of
-
A small molecule inhibitor of PTP1B and PTPN2 enhances T cell anti-tumor immunity.bioRxiv [Preprint]. 2023 Jun 28:2023.06.16.545220. doi: 10.1101/2023.06.16.545220. bioRxiv. 2023. Update in: Nat Commun. 2023 Jul 27;14(1):4524. doi: 10.1038/s41467-023-40170-8. PMID: 37397992 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

