Uptake and anti-inflammatory effects of liposomal astaxanthin on endothelial cells tracked by Raman and fluorescence imaging
- PMID: 37500736
- PMCID: PMC10374751
- DOI: 10.1007/s00604-023-05888-8
Uptake and anti-inflammatory effects of liposomal astaxanthin on endothelial cells tracked by Raman and fluorescence imaging
Abstract
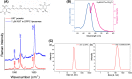
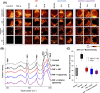
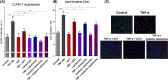
Astaxanthin (AXT) is a lipophilic antioxidant and anti-inflammatory natural pigment whose cellular uptake and bioavailability could be improved via liposomal encapsulation. Endothelial cells (EC) line the lumen of all blood vessels and are tasked with multiple roles toward maintaining cardiovascular homeostasis. Endothelial dysfunction is linked to the development of many diseases and is closely interconnected with oxidative stress and vascular inflammation. The uptake of free and liposomal AXT into EC was investigated using Raman and fluorescence microscopies. AXT was either encapsulated in neutral or cationic liposomes. Enhanced uptake and anti-inflammatory effects of liposomal AXT were observed. The anti-inflammatory effects of liposomal AXT were especially prominent in reducing EC lipid unsaturation, lowering numbers of lipid droplets (LDs), and decreasing intercellular adhesion molecule 1 (ICAM-1) overexpression, which is considered a well-known marker for endothelial inflammation. These findings highlight the benefits of AXT liposomal encapsulation on EC and the applicability of Raman imaging to investigate such effects.
Keywords: Carotenoids; Cellular uptake; Endothelium; Liposomes; Raman microscopy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Trif M, Craciunescu O. Nanotechnology and functional foods. Chichester, UK: John Wiley & Sons, Ltd; 2015. Liposome as efficient system for intracellular delivery of bioactive molecules; pp. 191–213.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
