Nonclassical mechanisms to irreversibly suppress β-hematin crystal growth
- PMID: 37500754
- PMCID: PMC10374632
- DOI: 10.1038/s42003-023-05046-z
Nonclassical mechanisms to irreversibly suppress β-hematin crystal growth
Abstract
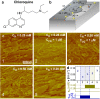
Hematin crystallization is an essential element of heme detoxification of malaria parasites and its inhibition by antimalarial drugs is a common treatment avenue. We demonstrate at biomimetic conditions in vitro irreversible inhibition of hematin crystal growth due to distinct cooperative mechanisms that activate at high crystallization driving forces. The evolution of crystal shape after limited-time exposure to both artemisinin metabolites and quinoline-class antimalarials indicates that crystal growth remains suppressed after the artemisinin metabolites and the drugs are purged from the solution. Treating malaria parasites with the same agents reveals that three- and six-hour inhibitor pulses inhibit parasite growth with efficacy comparable to that of inhibitor exposure during the entire parasite lifetime. Time-resolved in situ atomic force microscopy (AFM), complemented by light scattering, reveals two molecular-level mechanisms of inhibitor action that prevent β-hematin growth recovery. Hematin adducts of artemisinins incite copious nucleation of nonextendable nanocrystals, which incorporate into larger growing crystals, whereas pyronaridine, a quinoline-class drug, promotes step bunches, which evolve to engender abundant dislocations. Both incorporated crystals and dislocations are known to induce lattice strain, which persists and permanently impedes crystal growth. Nucleation, step bunching, and other cooperative behaviors can be amplified or curtailed as means to control crystal sizes, size distributions, aspect ratios, and other properties essential for numerous fields that rely on crystalline materials.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

