Development and validation of a multiplex electrochemiluminescence immunoassay to evaluate dry eye disease in rat tear fluids
- PMID: 37500810
- PMCID: PMC10374623
- DOI: 10.1038/s41598-023-39397-8
Development and validation of a multiplex electrochemiluminescence immunoassay to evaluate dry eye disease in rat tear fluids
Abstract
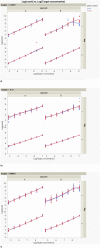
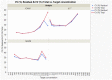
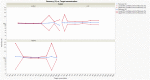
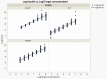
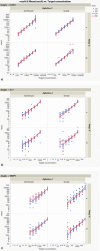
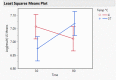
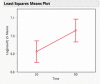
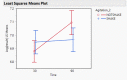
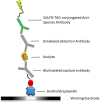
Dry eye disease (DED) is a challenge in ophthalmology. Rat models represent valuable tools to study the pathophysiology and to develop novel treatments. A major challenge in DED research is detecting multiple biomarkers in a low tear volume sample. Multiplex immunoassays for DED rat research are missing. We have developed a multiplex electrochemiluminescence immunoassay (ECLIA) to detect three biomarkers for DED: MMP-9, IL-17 and ICAM-1. Tears, used as matrix, were collected from six healthy Wistar rats. Assays were run based on the U-Plex Meso Scale Diagnostics (MSD) platform, by two independent operators according to the EMA guideline on bioanalytical method validation. Linear mixed, regression models were fit to perform the statistical analysis on the range of concentrations for the chosen analytes. During optimization, it has observed that incubation time, temperature and agitation affected the robustness of the protocol. ECLIA optimum conditions include the use of antibodies at 0.5 µg/ml concentration and 1 h incubation at room temperature with shaking. Precision met the acceptance criteria in the chosen range: 1062-133 pg/ml for ICAM-1, 275-34.4 pg/ml for IL-17, 1750-219 pg/ml for MMP-9. Accuracy and linearity were acceptable for a broader range. This is the first report of a validated ECLIA that allows measurements of three relevant DED biomarkers in rat tear fluids.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Matrix Metalloproteinase 9 Testing in Dry Eye Disease Using a Commercially Available Point-of-Care Immunoassay.Ophthalmology. 2016 Nov;123(11):2300-2308. doi: 10.1016/j.ophtha.2016.07.028. Epub 2016 Sep 21. Ophthalmology. 2016. PMID: 27665213
-
Dichotomous versus 5-scale grading system for the interpretation of the point-of-care immunoassay for tear matrix metalloproteinase-9 in dry eye.Sci Rep. 2023 Apr 13;13(1):6085. doi: 10.1038/s41598-023-32928-3. Sci Rep. 2023. PMID: 37055446 Free PMC article.
-
Study design and baseline findings from the progression of ocular findings (PROOF) natural history study of dry eye.BMC Ophthalmol. 2017 Dec 28;17(1):265. doi: 10.1186/s12886-017-0646-5. BMC Ophthalmol. 2017. PMID: 29284427 Free PMC article. Clinical Trial.
-
Definition and Diagnostic Criteria of Dry Eye Disease: Historical Overview and Future Directions.Invest Ophthalmol Vis Sci. 2018 Nov 1;59(14):DES7-DES12. doi: 10.1167/iovs.17-23475. Invest Ophthalmol Vis Sci. 2018. PMID: 30481800 Review.
-
Tear film assessments for the diagnosis of dry eye.Curr Opin Allergy Clin Immunol. 2016 Oct;16(5):487-91. doi: 10.1097/ACI.0000000000000307. Curr Opin Allergy Clin Immunol. 2016. PMID: 27585060 Review.
References
-
- Erickson S, Sullivan AG, Barabino S, Begovic E, Benitez-del-Castillo JM, Bonini S, Borges JS, Brzheskiy V, Bulat N, Cerim A, Craig JP, Cușnir V, Cușnir V, Doan S, Dülger E, Farrant S, Geerling G, Goldblum D, Golubev S, Sullivan DA. TFOS European Ambassador meeting: Unmet needs and future scientific and clinical solutions for ocular surface diseases. Ocul. Surf. 2020;18(4):936–962. doi: 10.1016/j.jtos.2020.05.006. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

